Sirtuins in kidney diseases: potential mechanism and therapeutic targets
- PMID: 38347622
- PMCID: PMC10860260
- DOI: 10.1186/s12964-023-01442-4
Sirtuins in kidney diseases: potential mechanism and therapeutic targets
Abstract
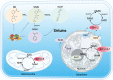
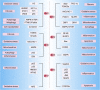
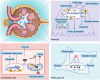
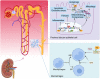
Sirtuins, which are NAD+-dependent class III histone deacetylases, are involved in various biological processes, including DNA damage repair, immune inflammation, oxidative stress, mitochondrial homeostasis, autophagy, and apoptosis. Sirtuins are essential regulators of cellular function and organismal health. Increasing evidence suggests that the development of age-related diseases, including kidney diseases, is associated with aberrant expression of sirtuins, and that regulation of sirtuins expression and activity can effectively improve kidney function and delay the progression of kidney disease. In this review, we summarise current studies highlighting the role of sirtuins in renal diseases. First, we discuss sirtuin family members and their main mechanisms of action. We then outline the possible roles of sirtuins in various cell types in kidney diseases. Finally, we summarise the compounds that activate or inhibit sirtuin activity and that consequently ameliorate renal diseases. In conclusion, targeted modulation of sirtuins is a potential therapeutic strategy for kidney diseases. Video Abstract.
Keywords: Endothelial cells; Kidney diseases; Macrophages; Podocyte; Renal tubular epithelial cells; Sirtuins.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

