The impact of kidney function on plasma neurofilament light and phospho-tau 181 in a community-based cohort: the Shanghai Aging Study
- PMID: 38347655
- PMCID: PMC10860286
- DOI: 10.1186/s13195-024-01401-2
The impact of kidney function on plasma neurofilament light and phospho-tau 181 in a community-based cohort: the Shanghai Aging Study
Abstract
Background: The blood-based biomarkers are approaching the clinical practice of Alzheimer's disease (AD). Chronic kidney disease (CKD) has a potential confounding effect on peripheral protein levels. It is essential to characterize the impact of renal function on AD markers.
Methods: Plasma phospho-tau181 (P-tau181), and neurofilament light (NfL) were assayed via the Simoa HD-X platform in 1189 dementia-free participants from the Shanghai Aging Study (SAS). The estimated glomerular filter rate (eGFR) was calculated. The association between renal function and blood NfL, P-tau181 was analyzed. An analysis of interactions between various demographic and comorbid factors and eGFR was conducted.
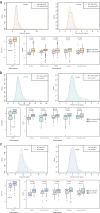
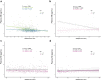
Results: The eGFR levels were negatively associated with plasma concentrations of NfL and P-tau181 (B = - 0.19, 95% CI - 0.224 to - 0.156, P < 0.001; B = - 0.009, 95% CI - 0.013 to -0.005, P < 0.001, respectively). After adjusting for demographic characteristics and comorbid diseases, eGFR remained significantly correlated with plasma NfL (B = - 0.010, 95% CI - 0.133 to - 0.068, P < 0.001), but not with P-tau181 (B = - 0.003, 95% CI - 0.007 to 0.001, P = 0.194). A significant interaction between age and eGFR was found for plasma NfL (Pinteraction < 0.001). In participants ≥ 70 years and with eGFR < 60 ml/min/1.73 m2, the correlation between eGFR and plasma NfL was significantly remarkable (B = - 0.790, 95% CI - 1.026 to - 0,554, P < 0.001).
Conclusions: Considering renal function and age is crucial when interpreting AD biomarkers in the general aging population.
Keywords: Comorbid conditions; Glomerular filter rate; Neurofilament light; Phospho-tau181; Renal function.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Wu X, Xiao Z, Yi J, Ding S, Gu H, Wu W, Luo J, Liang X, Zheng L, Xu H, et al. Development of a plasma biomarker diagnostic model incorporating ultrasensitive digital immunoassay as a screening strategy for Alzheimer disease in a Chinese population. Clin Chem. 2021;67(12):1628–1639. doi: 10.1093/clinchem/hvab192. - DOI - PubMed
-
- Benussi A, Cantoni V, Rivolta J, Archetti S, Micheli A, Ashton N, Zetterberg H, Blennow K, Borroni B. Classification accuracy of blood-based and neurophysiological markers in the differential diagnosis of Alzheimer's disease and frontotemporal lobar degeneration. Alzheimers Res Ther. 2022;14(1):155. doi: 10.1186/s13195-022-01094-5. - DOI - PMC - PubMed
-
- Karikari TK, Benedet AL, Ashton NJ, Lantero Rodriguez J, Snellman A, Suárez-Calvet M, Saha-Chaudhuri P, Lussier F, Kvartsberg H, Rial AM, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer's disease neuroimaging initiative. Mol Psychiatry. 2021;26(2):429–442. doi: 10.1038/s41380-020-00923-z. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

