Mechanistic Investigation, Wavelength-Dependent Reactivity, and Expanded Reactivity of N-Aryl Azacycle Photomediated Ring Contractions
- PMID: 38347659
- PMCID: PMC11646679
- DOI: 10.1021/jacs.3c13982
Mechanistic Investigation, Wavelength-Dependent Reactivity, and Expanded Reactivity of N-Aryl Azacycle Photomediated Ring Contractions
Abstract
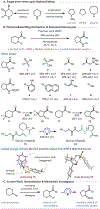
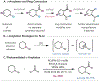
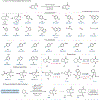
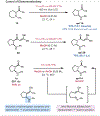
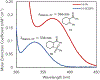
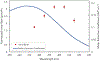
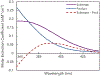
Under mild blue-light irradiation, α-acylated saturated heterocycles undergo a photomediated one-atom ring contraction that extrudes a heteroatom from the cyclic core. However, for nitrogenous heterocycles, this powerful skeletal edit has been limited to substrates bearing electron-withdrawing substituents on nitrogen. Moreover, the mechanism and wavelength-dependent efficiency of this transformation have remained unclear. In this work, we increased the electron richness of nitrogen in saturated azacycles to improve light absorption and strengthen critical intramolecular hydrogen bonding while enabling the direct installation of the photoreactive handle. As a result, a broadly expanded substrate scope, including underexplored electron-rich substrates and previously unsuccessful heterocycles, has now been achieved. The significantly improved yields and diastereoselectivities have facilitated reaction rate, kinetic isotope effect (KIE), and quenching studies, in addition to the determination of quantum yields. Guided by these studies, we propose a revised ET/PT mechanism for the ring contraction, which is additionally corroborated by computational characterization of the lowest-energy excited states of α-acylated substrates through time-dependent DFT. The efficiency of the ring contraction at wavelengths longer than those strongly absorbed by the substrates was investigated through wavelength-dependent rate measurements, which revealed a red shift of the photochemical action plot relative to substrate absorbance. The elucidated mechanistic and photophysical details effectively rationalize empirical observations, including additive effects, that were previously poorly understood. Our findings not only demonstrate enhanced synthetic utility of the photomediated ring contraction and shed light on mechanistic details but may also offer valuable guidance for understanding wavelength-dependent reactivity for related photochemical systems.
Figures
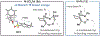



Similar articles
-
Interrogation of Enantioselectivity in the Photomediated Ring Contractions of Saturated Heterocycles.J Am Chem Soc. 2025 Jan 15;147(2):1851-1866. doi: 10.1021/jacs.4c13999. Epub 2025 Jan 2. J Am Chem Soc. 2025. PMID: 39746148 Free PMC article.
-
Photomediated ring contraction of saturated heterocycles.Science. 2021 Aug 27;373(6558):1004-1012. doi: 10.1126/science.abi7183. Epub 2021 Aug 12. Science. 2021. PMID: 34385352 Free PMC article.
-
Ring-strain-enabled reaction discovery: new heterocycles from bicyclo[1.1.0]butanes.Acc Chem Res. 2015 Apr 21;48(4):1149-58. doi: 10.1021/ar500437h. Epub 2015 Mar 16. Acc Chem Res. 2015. PMID: 25775119
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
-
Alkynoates as Versatile and Powerful Chemical Tools for the Rapid Assembly of Diverse Heterocycles under Transition-Metal Catalysis: Recent Developments and Challenges.Top Curr Chem (Cham). 2021 Jan 5;379(1):3. doi: 10.1007/s41061-020-00316-4. Top Curr Chem (Cham). 2021. PMID: 33398642 Review.
Cited by
-
Selective nitrogen insertion into aryl alkanes.Nat Commun. 2024 Jul 17;15(1):6016. doi: 10.1038/s41467-024-50383-0. Nat Commun. 2024. PMID: 39019881 Free PMC article.
-
Interrogation of Enantioselectivity in the Photomediated Ring Contractions of Saturated Heterocycles.J Am Chem Soc. 2025 Jan 15;147(2):1851-1866. doi: 10.1021/jacs.4c13999. Epub 2025 Jan 2. J Am Chem Soc. 2025. PMID: 39746148 Free PMC article.
-
Catalyst-free and wavelength-tuned glycosylation based on excited-state intramolecular proton transfer.Nat Commun. 2024 Nov 7;15(1):9661. doi: 10.1038/s41467-024-54020-8. Nat Commun. 2024. PMID: 39511172 Free PMC article.
-
Introducing Photochemical Action Plots as a Tool for Unlocking On-Off Switchable Behavior in a Polymeric Eosin Y Photocatalyst.Angew Chem Int Ed Engl. 2025 Jul;64(27):e202502890. doi: 10.1002/anie.202502890. Epub 2025 May 12. Angew Chem Int Ed Engl. 2025. PMID: 40302415 Free PMC article.
-
Precision Photochemistry: Every Photon Counts.Angew Chem Int Ed Engl. 2025 Aug 25;64(35):e202502651. doi: 10.1002/anie.202502651. Epub 2025 Aug 4. Angew Chem Int Ed Engl. 2025. PMID: 40755360 Free PMC article.
References
-
- Castellino NJ; Montgomery AP; Danon JJ; Kassiou M Late-Stage Functionalization for Improving Drug-like Molecular Properties. Chem. Rev 2023, 123, 8127–8153. - PubMed
-
- Peplow M. Skeleton Crew. Nature 2023, 618, 21–24. - PubMed
-
- Campos KR; Coleman PJ; Alvarez JC; Dreher SD; Garbaccio RM; Terrett NK; Tillyer RD; Truppo MD; Parmee ER The Importance of Synthetic Chemistry in the Pharmaceutical Industry. Science 2019, 363, No. eaat0805. - PubMed
-
- Qureshi MH; Williams R; Marshall C; Njardarson JT Top 200 Small Molecule Pharmaceuticals by Retail Sales in 2021. J. Chem. Educ 2010, 87, 1348.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

