Delayed-Onset Muscle Soreness Alters Mechanical Sensitivity, but Not Thermal Sensitivity or Pain Modulatory Function
- PMID: 38347855
- PMCID: PMC10860815
- DOI: 10.2147/JPR.S449787
Delayed-Onset Muscle Soreness Alters Mechanical Sensitivity, but Not Thermal Sensitivity or Pain Modulatory Function
Abstract
Introduction: Many clinical musculoskeletal pain conditions are characterized by chronic inflammation that sensitizes nociceptors. An unresolved issue is whether inflammation affects all nociceptors in a similar manner. Exercise-induced muscle damage (EIMD) has been proposed as a model for simulating clinical inflammatory pain in healthy samples. We sought to test the effect of EIMD on various painful stimuli (pressure and thermal), central pain processing (via the nociceptive flexion reflex) and endogenous pain modulation via conditioned pain modulation and exercise-induced hypoalgesia.
Methods: Eighteen participants (9F, age: 24.6 ± 3.3) were recruited for repeated measures testing and each completed pain sensitivity testing prior to and 48 hours after an eccentric exercise protocol. The participants performed a minimum of 6 rounds of 10 eccentric knee extension exercises to induce muscle damage and localized inflammation in the right quadriceps. Force decrements, knee range-of-motion, and delayed onset muscle soreness (DOMS) were used to quantify EIMD.
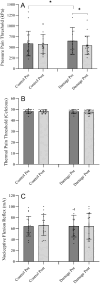
Results: There was a significant main effect of time for pressure pain (%diff; -58.9 ± 23.1; p = 0.02, ηp2 = 0.28) but no significant main effect was observed for limb (%diff; -15.5 ± 23.9; p = 0.53, ηp2 = 0.02). In contrast, there was a significant interaction between time and limb (p < 0.001, ηp2 = 0.47) whereby participants had lower pressure pain sensitivity in the right leg only after the damage protocol (%diff; -105.9 ± 29.2; p = 0.002).
Discussion: Individuals with chronic inflammatory pain usually have an increased sensitivity to pressure, thermal, and electrical stimuli, however, our sample, following muscle damage to induce acute inflammation only had sensitivity to mechanical pain. Exercise induced inflammation may reflect a peripheral sensitivity localized to the damaged muscle rather than a global sensitivity like those with chronic pain display.
Keywords: acute inflammation; exercise induced muscle damage; pain modulation; pain sensitivity.
© 2024 Peterson et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures

Similar articles
-
Local and Generalized Endogenous Pain Modulation in Healthy Men: Effects of Exercise and Exercise-Induced Muscle Damage.Pain Med. 2016 Dec;17(12):2422-2433. doi: 10.1093/pm/pnw077. Epub 2016 Jun 27. Pain Med. 2016. PMID: 28025376
-
BILATERAL SENSORY DEFICITS AND WIDESPREAD HYPERALGESIA OCCUR FOLLOWING INDUCED DELAYED ONSET MUSCLE SORENESS OF THE QUADRICEPS.Int J Sports Phys Ther. 2020 Feb;15(1):12-21. Int J Sports Phys Ther. 2020. PMID: 32089954 Free PMC article.
-
Enhanced temporal summation of pressure pain in the trapezius muscle after delayed onset muscle soreness.Exp Brain Res. 2006 Apr;170(2):182-90. doi: 10.1007/s00221-005-0196-6. Epub 2005 Nov 23. Exp Brain Res. 2006. PMID: 16328284
-
Effects of Pre-exercise Acute Vibration Training on Symptoms of Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis.J Strength Cond Res. 2022 Aug 1;36(8):2339-2348. doi: 10.1519/JSC.0000000000003789. Epub 2020 Aug 12. J Strength Cond Res. 2022. PMID: 32796411
-
The use of BCAA to decrease delayed-onset muscle soreness after a single bout of exercise: a systematic review and meta-analysis.Amino Acids. 2021 Nov;53(11):1663-1678. doi: 10.1007/s00726-021-03089-2. Epub 2021 Oct 20. Amino Acids. 2021. PMID: 34669012
Cited by
-
The deficient CLEC5A ameliorates the behavioral and pathological deficits via the microglial Aβ clearance in Alzheimer's disease mouse model.J Neuroinflammation. 2024 Oct 23;21(1):273. doi: 10.1186/s12974-024-03253-x. J Neuroinflammation. 2024. PMID: 39443966 Free PMC article.
References
-
- Fernandes JC, Martel‐Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(12):237–246. - PubMed
LinkOut - more resources
Full Text Sources

