Curcuma longa extract reduces serum inflammatory markers and postprandial hyperglycemia in healthy but borderline participants with overweight and glycemia in the normal/prediabetes range: a randomized, double-blind, and placebo-controlled trial
- PMID: 38347961
- PMCID: PMC10859506
- DOI: 10.3389/fnut.2024.1324196
Curcuma longa extract reduces serum inflammatory markers and postprandial hyperglycemia in healthy but borderline participants with overweight and glycemia in the normal/prediabetes range: a randomized, double-blind, and placebo-controlled trial
Abstract
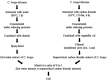
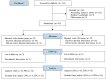
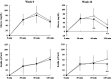
The spice turmeric, which has the Latin name Curcuma longa (C. longa), has various physiological effects. This study evaluated the effects of a hot water mixture with supercritical carbon dioxide C. longa extracts, CLE, and the potential active components of C. longa, turmeronols A and B and bisacurone on inflammation and glucose metabolism. First, we investigated the effect of CLE and the potential active components of C. longa on lipopolysaccharide-induced inflammation in RAW264.7 macrophages. We found a significant decrease in the production of interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and nitric oxide with CLE, turmeronol A, and bisacurone, Significant inhibition of each of these substances was also observed, except for TNF-α with turmeronol B. The second part of our work was a 12-week randomized, double-blind, placebo-controlled study in healthy but borderline adults aged 40 to 69 years with overweight and normal/prediabetes glycemia. We compared blood inflammatory and glycometabolic markers in the CLE (n = 55) and placebo groups (n = 55). We found significantly lower serum high-sensitivity C-reactive protein and hemoglobin A1c levels in the CLE group. This group also showed significant improvements in postprandial hyperglycemia and insulin sensitivity indices. Our findings indicate that CLE may reduce low-grade inflammation and thus improve insulin sensitivity and postprandial hyperglycemia. Clinical trial registration: https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000051492, UMIN-CTR, UMIN000045106.
Keywords: C-reactive protein; Curcuma longa (turmeric) extract; bisacurone; chronic inflammation; hemoglobin A1c; insulin sensitivity; oral glucose tolerance test; turmeronol.
Copyright © 2024 Uchio, Okuda-Hanafusa, Sakaguchi, Saji, Muroyama, Murosaki, Yamamoto and Hirose.
Conflict of interest statement
RU, CO-H, HS, RS, KM, SM, YY, and YH are employed by House Wellness Foods Corp., which markets health food products. This study was funded by House Wellness Foods Corp. (Itami, Japan) and was conducted by a contract research organization (CRO; CPCC Co., Ltd., Tokyo, Japan). The CRO performed the data collection and analysis. The funder designed this study, supplied the tests capsules, prepared the manuscript, and decided to submit it for publication. The article processing charges were also funded by House Wellness Foods Corp.
Figures
Similar articles
-
Curcuma longa extract improves serum inflammatory markers and mental health in healthy participants who are overweight: a randomized, double-blind, placebo-controlled trial.Nutr J. 2021 Nov 13;20(1):91. doi: 10.1186/s12937-021-00748-8. Nutr J. 2021. PMID: 34774052 Free PMC article. Clinical Trial.
-
A Hot Water Extract of Curcuma longa L. Improves Fasting Serum Glucose Levels in Participants with Low-Grade Inflammation: Reanalysis of Data from Two Randomized, Double-Blind, Placebo-Controlled Trials.Nutrients. 2022 Sep 13;14(18):3763. doi: 10.3390/nu14183763. Nutrients. 2022. PMID: 36145139 Free PMC article. Clinical Trial.
-
Hot Water Extract of Curcuma longa L. Improves Serum Inflammatory Markers and General Health in Subjects with Overweight or Prehypertension/Mild Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial.Nutrients. 2019 Aug 7;11(8):1822. doi: 10.3390/nu11081822. Nutrients. 2019. PMID: 31394768 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial.Front Immunol. 2022 Jul 22;13:891822. doi: 10.3389/fimmu.2022.891822. eCollection 2022. Front Immunol. 2022. PMID: 35935936 Free PMC article.
-
Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review.Biofactors. 2021 May;47(3):311-350. doi: 10.1002/biof.1716. Epub 2021 Feb 19. Biofactors. 2021. PMID: 33606322 Review.
Cited by
-
Dietary Interventions, Supplements, and Plant-Derived Compounds for Adjunct Vitiligo Management: A Review of the Literature.Nutrients. 2025 Jan 20;17(2):357. doi: 10.3390/nu17020357. Nutrients. 2025. PMID: 39861486 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

