The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy
- PMID: 38348027
- PMCID: PMC10859531
- DOI: 10.3389/fimmu.2024.1331641
The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy
Abstract
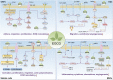
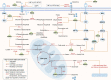
Cancer, a disease that modern medicine has not fully understood and conquered, with its high incidence and mortality, deprives countless patients of health and even life. According to global cancer statistics, there were an estimated 19.3 million new cancer cases and nearly 10 million cancer deaths in 2020, with the age-standardized incidence and mortality rates of 201.0 and 100.7 per 100,000, respectively. Although remarkable advancements have been made in therapeutic strategies recently, the overall prognosis of cancer patients remains not optimistic. Consequently, there are still many severe challenges to be faced and difficult problems to be solved in cancer therapy today. Epigallocatechin gallate (EGCG), a natural polyphenol extracted from tea leaves, has received much attention for its antitumor effects. Accumulating investigations have confirmed that EGCG can inhibit tumorigenesis and progression by triggering apoptosis, suppressing proliferation, invasion, and migration, altering tumor epigenetic modification, and overcoming chemotherapy resistance. Nevertheless, its regulatory roles and biomolecular mechanisms in the immune microenvironment, metabolic microenvironment, and immunotherapy remain obscure. In this article, we summarized the most recent updates about the effects of EGCG on tumor microenvironment (TME), metabolic reprogramming, and anti-cancer immunotherapy. The results demonstrated EGCG can promote the anti-cancer immune response of cytotoxic lymphocytes and dendritic cells (DCs), attenuate the immunosuppression of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), and inhibit the tumor-promoting functions of tumor-associated macrophages (TAMs), tumor-associated neutrophils (TANs), and various stromal cells including cancer-associated fibroblasts (CAFs), endothelial cells (ECs), stellate cells, and mesenchymal stem/stromal cells (MSCs). Additionally, EGCG can suppress multiple metabolic reprogramming pathways, including glucose uptake, aerobic glycolysis, glutamine metabolism, fatty acid anabolism, and nucleotide synthesis. Finally, EGCG, as an immunomodulator and immune checkpoint blockade, can enhance immunotherapeutic efficacy and may be a promising candidate for antitumor immunotherapy. In conclusion, EGCG plays versatile regulatory roles in TME and metabolic reprogramming, which provides novel insights and combined therapeutic strategies for cancer immunotherapy.
Keywords: antitumor immunity; epigallocatechin gallate; immunotherapy; metabolic reprogramming; tumor microenvironment.
Copyright © 2024 Li, Cao, Sun, Cui, Zhang, Jiang and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Involvement of tumor immune microenvironment metabolic reprogramming in colorectal cancer progression, immune escape, and response to immunotherapy.Front Immunol. 2024 Jul 25;15:1353787. doi: 10.3389/fimmu.2024.1353787. eCollection 2024. Front Immunol. 2024. PMID: 39119332 Free PMC article. Review.
-
Metabolic Reprogramming of Immune Cells in the Tumor Microenvironment.Int J Mol Sci. 2024 Nov 14;25(22):12223. doi: 10.3390/ijms252212223. Int J Mol Sci. 2024. PMID: 39596288 Free PMC article. Review.
-
Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis.Acta Biomater. 2021 Nov;135:567-581. doi: 10.1016/j.actbio.2021.09.003. Epub 2021 Sep 8. Acta Biomater. 2021. PMID: 34506976
-
Metal-phenolic networks reverse the immunosuppressive tumor microenvironment via dual metabolism regulation and immunogenic cell death.J Control Release. 2025 Jul 10;383:113775. doi: 10.1016/j.jconrel.2025.113775. Epub 2025 Apr 26. J Control Release. 2025. PMID: 40294797
-
Metabolic reprogramming and crosstalk of cancer-related fibroblasts and immune cells in the tumor microenvironment.Front Endocrinol (Lausanne). 2022 Aug 15;13:988295. doi: 10.3389/fendo.2022.988295. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36046791 Free PMC article. Review.
Cited by
-
Recent advances in understanding the immune microenvironment in ovarian cancer.Front Immunol. 2024 Jun 5;15:1412328. doi: 10.3389/fimmu.2024.1412328. eCollection 2024. Front Immunol. 2024. PMID: 38903506 Free PMC article. Review.
-
Phytochemicals as Chemo-Preventive and Therapeutic Agents Against Bladder Cancer: A Comprehensive Review.Diseases. 2025 Mar 30;13(4):103. doi: 10.3390/diseases13040103. Diseases. 2025. PMID: 40277814 Free PMC article. Review.
-
Integrative pan-cancer analysis and experiment validation identified GLS as a biomarker in tumor progression, prognosis, immune microenvironment, and immunotherapy.Sci Rep. 2025 Jan 2;15(1):525. doi: 10.1038/s41598-024-84916-w. Sci Rep. 2025. PMID: 39747578 Free PMC article.
-
Unlocking the adenosine receptor mechanism of the tumour immune microenvironment.Front Immunol. 2024 Jun 27;15:1434118. doi: 10.3389/fimmu.2024.1434118. eCollection 2024. Front Immunol. 2024. PMID: 38994361 Free PMC article. Review.
-
Dietary factors and their influence on immunotherapy strategies in oncology: a comprehensive review.Cell Death Dis. 2024 Apr 9;15(4):254. doi: 10.1038/s41419-024-06641-6. Cell Death Dis. 2024. PMID: 38594256 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

