Monocyte-derived alveolar macrophages are key drivers of smoke-induced lung inflammation and tissue remodeling
- PMID: 38348034
- PMCID: PMC10859862
- DOI: 10.3389/fimmu.2024.1325090
Monocyte-derived alveolar macrophages are key drivers of smoke-induced lung inflammation and tissue remodeling
Abstract
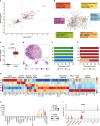
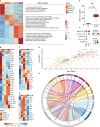
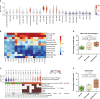
Smoking is a leading risk factor of chronic obstructive pulmonary disease (COPD), that is characterized by chronic lung inflammation, tissue remodeling and emphysema. Although inflammation is critical to COPD pathogenesis, the cellular and molecular basis underlying smoking-induced lung inflammation and pathology remains unclear. Using murine smoke models and single-cell RNA-sequencing, we show that smoking establishes a self-amplifying inflammatory loop characterized by an influx of molecularly heterogeneous neutrophil subsets and excessive recruitment of monocyte-derived alveolar macrophages (MoAM). In contrast to tissue-resident AM, MoAM are absent in homeostasis and characterized by a pro-inflammatory gene signature. Moreover, MoAM represent 46% of AM in emphysematous mice and express markers causally linked to emphysema. We also demonstrate the presence of pro-inflammatory and tissue remodeling associated MoAM orthologs in humans that are significantly increased in emphysematous COPD patients. Inhibition of the IRAK4 kinase depletes a rare inflammatory neutrophil subset, diminishes MoAM recruitment, and alleviates inflammation in the lung of cigarette smoke-exposed mice. This study extends our understanding of the molecular signaling circuits and cellular dynamics in smoking-induced lung inflammation and pathology, highlights the functional consequence of monocyte and neutrophil recruitment, identifies MoAM as key drivers of the inflammatory process, and supports their contribution to pathological tissue remodeling.
Keywords: COPD; IRAK4 inhibitor; lung inflammation; monocyte-derived alveolar macrophages; neutrophils; single-cell RNA-sequencing; smoking; tissue remodeling.
Copyright © 2024 Wohnhaas, Baßler, Watson, Shen, Leparc, Tilp, Heinemann, Kind, Stierstorfer, Delić, Brunner, Gantner, Schultze, Viollet and Baum.
Conflict of interest statement
Author FG was employed by company C. H. Boehringer Sohn AG & Co. KG. CW, CWa, YS, GL, CT, FH, DK, BS, DD, CV, and PB were employed by Boehringer Ingelheim Pharma GmbH & Co. KG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures



Similar articles
-
Inflammatory Cellular Response to Mechanical Ventilation in Elastase-Induced Experimental Emphysema: Role of Preexisting Alveolar Macrophages Infiltration.Biomed Res Int. 2018 Dec 19;2018:5721293. doi: 10.1155/2018/5721293. eCollection 2018. Biomed Res Int. 2018. PMID: 30662910 Free PMC article.
-
Inflammatory cells and chronic obstructive pulmonary disease.Curr Drug Targets Inflamm Allergy. 2005 Dec;4(6):607-18. doi: 10.2174/156801005774912824. Curr Drug Targets Inflamm Allergy. 2005. PMID: 17305517 Review.
-
Lysophospholipid Acyltransferase 9 Promotes Emphysema Formation via Platelet-activating Factor.Am J Respir Cell Mol Biol. 2024 Jun;70(6):482-492. doi: 10.1165/rcmb.2023-0253OC. Am J Respir Cell Mol Biol. 2024. PMID: 38377392
-
Vitamin E isoform γ-tocotrienol protects against emphysema in cigarette smoke-induced COPD.Free Radic Biol Med. 2017 Sep;110:332-344. doi: 10.1016/j.freeradbiomed.2017.06.023. Epub 2017 Jul 3. Free Radic Biol Med. 2017. PMID: 28684161
-
Increased surfactant protein-D and foamy macrophages in smoking-induced mouse emphysema.Respirology. 2007 Mar;12(2):191-201. doi: 10.1111/j.1440-1843.2006.01009.x. Respirology. 2007. PMID: 17298450 Review.
Cited by
-
Advances on the Role of Lung Macrophages in the Pathogenesis of Chronic Obstructive Pulmonary Disease in the Era of Single-Cell Genomics.Int J Med Sci. 2025 Jan 1;22(2):298-308. doi: 10.7150/ijms.100160. eCollection 2025. Int J Med Sci. 2025. PMID: 39781522 Free PMC article. Review.
-
BPIFB4 rs4339026 A > G polymorphism impacts COPD susceptibility in the Kashi population, China.Sci Rep. 2025 Apr 25;15(1):14515. doi: 10.1038/s41598-025-98599-4. Sci Rep. 2025. PMID: 40280985 Free PMC article.
-
Differences in glycolytic metabolism between tissue-resident alveolar macrophages and recruited lung macrophages.Front Immunol. 2025 Feb 28;16:1535796. doi: 10.3389/fimmu.2025.1535796. eCollection 2025. Front Immunol. 2025. PMID: 40092977 Free PMC article. Review.
-
A next-generation system for smoke inhalation integrated with a breathing lung-on-chip to model human lung responses to cigarette exposure.Sci Rep. 2025 May 25;15(1):18181. doi: 10.1038/s41598-025-00438-z. Sci Rep. 2025. PMID: 40414911 Free PMC article.
-
Transcriptomic analysis reveals distinct effects of cigarette smoke on murine airspace and bone-marrow derived macrophages.Respir Res. 2024 Aug 24;25(1):322. doi: 10.1186/s12931-024-02939-3. Respir Res. 2024. PMID: 39182076 Free PMC article.
References
-
- Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, et al. . Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med (2022) 10:447–58. doi: 10.1016/s2213-2600(21)00511-7 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

