Roles of tumor necrosis factor-like ligand 1A in γδT-cell activation and psoriasis pathogenesis
- PMID: 38348035
- PMCID: PMC10859483
- DOI: 10.3389/fimmu.2024.1340467
Roles of tumor necrosis factor-like ligand 1A in γδT-cell activation and psoriasis pathogenesis
Abstract
Background: Interleukin (IL)-17-producing γδT (γδT17) cells mediate inflammatory responses in barrier tissues. Dysregulated γδT17 cell activation can lead to the overproduction of IL-17 and IL-22 and the development of inflammatory diseases, including psoriasis. IL-23 and IL-1β are known to synergistically activate γδT17 cells, but the regulatory mechanisms of γδT17 cells have not been fully elucidated. This study aimed to reveal the contribution of the inflammatory cytokine tumor necrosis factor-like ligand 1A (TL1A) to γδT17 cell activation and psoriasis development.
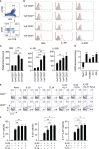
Methods: Anti-TL1A antibody was injected into an imiquimod (IMQ)-induced murine psoriasis model. TL1A receptor expression was analyzed in splenic and dermal γδT cells. γδT cells were tested for cytokine production in vitro and in vivo under stimulation with IL-23, IL-1β, and TL1A. TL1A was applied to a psoriasis model induced by intradermal IL-23 injection. Mice deficient in γδT cells were intradermally injected with IL-23 plus TL1A to verify the contribution of TL1A-dependent γδT-cell activation to psoriasis development.
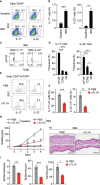
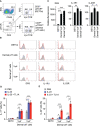
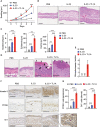
Results: Neutralization of TL1A attenuated γδT17 cell activation in IMQ-treated skin. TL1A induced cytokine production by splenic γδT17 cells in synergy with IL-23. Dermal γδT17 cells constitutively expressed a TL1A receptor at high levels and vigorously produced IL-22 upon intradermal IL-23 and TL1A injection but not IL-23 alone. TL1A exacerbated the dermal symptoms induced by IL-23 injection in wild-type but not in γδT cell-deficient mice.
Conclusion: These findings suggest a novel regulatory mechanism of γδT cells through TL1A and its involvement in psoriasis pathogenesis as a possible therapeutic target.
Keywords: IL-17; IL-22; TL1A; cytokine; psoriasis; γδ T cells.
Copyright © 2024 Wang, Kozai, Hiraishi, Rubel, Ichii, Inaba, Matsuo and Takada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effects of tumor necrosis factor-like ligand 1A (TL1A) on imiquimod-induced psoriasiform skin inflammation in mice.Arch Dermatol Res. 2020 Sep;312(7):481-490. doi: 10.1007/s00403-019-02030-8. Epub 2020 Jan 18. Arch Dermatol Res. 2020. PMID: 31953572
-
REV-ERB agonist suppresses IL-17 production in γδT cells and improves psoriatic dermatitis in a mouse model.Biomed Pharmacother. 2021 Dec;144:112283. doi: 10.1016/j.biopha.2021.112283. Epub 2021 Oct 7. Biomed Pharmacother. 2021. PMID: 34628169
-
Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses.Proc Natl Acad Sci U S A. 2015 Jun 30;112(26):8046-51. doi: 10.1073/pnas.1508990112. Epub 2015 Jun 15. Proc Natl Acad Sci U S A. 2015. PMID: 26080440 Free PMC article.
-
Hyperforin Ameliorates Imiquimod-Induced Psoriasis-Like Murine Skin Inflammation by Modulating IL-17A-Producing γδ T Cells.Front Immunol. 2021 May 5;12:635076. doi: 10.3389/fimmu.2021.635076. eCollection 2021. Front Immunol. 2021. PMID: 34025642 Free PMC article.
-
Gamma Delta T Cells and Their Pathogenic Role in Psoriasis.Front Immunol. 2021 Feb 25;12:627139. doi: 10.3389/fimmu.2021.627139. eCollection 2021. Front Immunol. 2021. PMID: 33732249 Free PMC article. Review.
Cited by
-
Antibodies Targeting the Tumor Necrosis Factor-Like Ligand 1A in Inflammatory Bowel Disease: A New Kid on the (Biologics) Block?Digestion. 2024;105(6):411-418. doi: 10.1159/000540421. Epub 2024 Jul 26. Digestion. 2024. PMID: 39068930 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

