Study protocol of a double-blind randomized control trial of transcranial direct current stimulation in post-stroke fatigue
- PMID: 38348114
- PMCID: PMC10860680
- DOI: 10.3389/fneur.2023.1297429
Study protocol of a double-blind randomized control trial of transcranial direct current stimulation in post-stroke fatigue
Abstract
Rationale: Post-stroke fatigue (PSF) is a frequent problem in stroke survivors and often hinders their rehabilitation. PSF is difficult to treat, and pharmacological therapy is often ineffective. Transcranial direct current stimulation (tDCS) can modulate motor, sensory, cognitive and behavioral responses, as it alters neuronal activity by delivering a small amount of current via the scalp to the cortex, resulting in prolonged alterations to brain function. tDCS has been studied for the treatment of fatigue associated with other neurological diseases, namely, multiple sclerosis, Parkinson's disease and post-polio syndrome.
Aims: This proposed project will examine the effect of tDCS on PSF.
Sample size estimates: We will recruit 156 participants aged 18 to 80 with chronic stroke and allocate them equally to two groups (i.e., n = 78 per group).
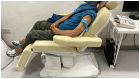
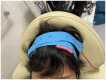
Methods and design: This proposed project will be a double-blind randomized control trial. The participants will be randomly divided into two groups. The control group will receive sham tDCS, and the treatment group will receive active tDCS. The latter treatment will involve application of a constant 2-mA current via one 5 × 5-cm anodal electrode positioned on the scalp over the C3 or C4 positions (motor cortex) of the lesioned hemisphere and one cathodal electrode positioned at the ipsilateral shoulder in two 20-min sessions per day for 5 days. The period of follow-up will be 4 weeks.
Study outcomes: The primary outcome measure will be a change in fatigue severity, as measured using the modified fatigue impact scale (MFIS). The participants' scores on the MFIS (total score and physical, cognitive and psychosocial subscores) will be collected before treatment (T0), after 10 treatment sessions, i.e., 1 day after the fifth treatment day (T1), and 1 week (T2), 2 weeks (T3) and 4 weeks (T4) thereafter. Both per-protocol analysis and intention-to-treat analysis will be performed.
Discussion: This proposed project will provide proof-of-concept, i.e., demonstrate the benefits of tDCS for the treatment of PSF. The beneficiaries are the subjects participated in the study. This will stimulate further research to optimize tDCS parameters for the treatment of PSF.
Clinical trial registration: www.Chictr.org.cn, identifier: ChiCTR2100052515.
Keywords: post-stroke fatigue (PSF); randomized control trial (RCT); rehabilitation; stroke; transcranial direct current stimulation (tDCS).
Copyright © 2024 Tang, Lu, Leung, Kim and Fong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effects of transcranial direct current stimulation on patients with post-stroke fatigue: a study protocol for a double-blind randomized controlled trial.Trials. 2022 Mar 5;23(1):200. doi: 10.1186/s13063-022-06128-9. Trials. 2022. PMID: 35248120 Free PMC article.
-
Combining transcranial direct current stimulation with music therapy improves cognitive function in schizophrenia: study protocol for a randomized, double-blind, sham-controlled clinical trial.Front Psychiatry. 2025 May 8;16:1543789. doi: 10.3389/fpsyt.2025.1543789. eCollection 2025. Front Psychiatry. 2025. PMID: 40417274 Free PMC article.
-
High-density transcranial direct current stimulation to improve upper limb motor function following stroke: study protocol for a double-blind randomized clinical trial targeting prefrontal and/or cerebellar cognitive contributions to voluntary motion.Trials. 2023 Dec 4;24(1):783. doi: 10.1186/s13063-023-07680-8. Trials. 2023. PMID: 38049806 Free PMC article.
-
Transcranial direct current stimulation for fatigue in neurological conditions: A systematic scoping review.Physiother Res Int. 2024 Jan;29(1):e2054. doi: 10.1002/pri.2054. Epub 2023 Oct 15. Physiother Res Int. 2024. PMID: 37838979
-
No add-on effect of tDCS on fatigue and depression in chronic stroke patients: A randomized sham-controlled trial combining tDCS with computerized cognitive training.Brain Behav. 2022 Jul;12(7):e2643. doi: 10.1002/brb3.2643. Epub 2022 Jun 6. Brain Behav. 2022. PMID: 35666655 Free PMC article. Review.
Cited by
-
Behavioral and Neural correlates of Post-STROKE Fatigue: A randomized controlled trial protocol.PLoS One. 2025 Jun 6;20(6):e0324591. doi: 10.1371/journal.pone.0324591. eCollection 2025. PLoS One. 2025. PMID: 40478794 Free PMC article.
References
-
- Rammohan KW, Rosenberg JH, Lynn DJ, Blumenfeld AM, Pollak CP, Nagaraja HN. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two Centre phase 2 study. J Neurol Neurosurg Psychiatry. (2002) 72:179–83. doi: 10.1136/jnnp.72.2.179, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous