Human iPSC-derived neurons reveal NMDAR-independent dysfunction following HIV-associated insults
- PMID: 38348237
- PMCID: PMC10859444
- DOI: 10.3389/fnmol.2023.1353562
Human iPSC-derived neurons reveal NMDAR-independent dysfunction following HIV-associated insults
Abstract
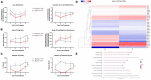
The central nervous system encounters a number of challenges following HIV infection, leading to increased risk for a collection of neurocognitive symptoms clinically classified as HIV-associated neurocognitive disorders (HAND). Studies attempting to identify causal mechanisms and potential therapeutic interventions have historically relied on primary rodent neurons, but a number of recent reports take advantage of iPSC-derived neurons in order to study these mechanisms in a readily reproducible, human model. We found that iPSC-derived neurons differentiated via an inducible neurogenin-2 transcription factor were resistant to gross toxicity from a number of HIV-associated insults previously reported to be toxic in rodent models, including HIV-infected myeloid cell supernatants and the integrase inhibitor antiretroviral drug, elvitegravir. Further examination of these cultures revealed robust resistance to NMDA receptor-mediated toxicity. We then performed a comparative analysis of iPSC neurons exposed to integrase inhibitors and activated microglial supernatants to study sub-cytotoxic alterations in micro electrode array (MEA)-measured neuronal activity and gene expression, identifying extracellular matrix interaction/morphogenesis as the most consistently altered pathways across HIV-associated insults. These findings illustrate that HIV-associated insults dysregulate human neuronal activity and organization even in the absence of gross NMDA-mediated neurotoxicity, which has important implications on the effects of these insults in neurodevelopment and on the interpretation of primary vs. iPSC in vitro neuronal studies.
Keywords: HIV; NMDA; antiretroviral therapy; iPSC; microglia; neuron.
Copyright © 2024 Starr, Nickoloff-Bybel, Abedalthaqafi, Albloushi and Jordan-Sciutto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Akay C., Lindl K. A., Shyam N., Nabet B., Goenaga-Vazquez Y., Ruzbarsky J., et al. (2012). Activation status of integrated stress response pathways in neurones and astrocytes of HIV-associated neurocognitive disorders (HAND) cortex. Neuropathol. Appl. Neurobiol. 38 175–200. 10.1111/j.1365-2990.2011.01215.x - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

