Home noninvasive ventilation in severe COPD: in whom does it work and how?
- PMID: 38348241
- PMCID: PMC10860207
- DOI: 10.1183/23120541.00600-2023
Home noninvasive ventilation in severe COPD: in whom does it work and how?
Abstract
Background: Not all hypercapnic COPD patients benefit from home noninvasive ventilation (NIV), and mechanisms through which NIV improves clinical outcomes remain uncertain. We aimed to identify "responders" to home NIV, denoted by a beneficial effect of NIV on arterial partial pressure of carbon dioxide (PaCO2), health-related quality of life (HRQoL) and survival, and investigated whether NIV achieves its beneficial effect through an improved PaCO2.
Methods: We used individual patient data from previous published trials collated for a systematic review. Linear mixed-effect models were conducted to compare the effect of NIV on PaCO2, HRQoL and survival, within subgroups defined by patient and treatment characteristics. Secondly, we conducted a causal mediation analysis to investigate whether the effect of NIV is mediated by a change in PaCO2.
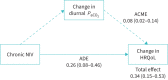
Findings: Data of 1142 participants from 16 studies were used. Participants treated with lower pressure support (<14 versus ≥14 cmH2O) and with lower adherence (<5 versus ≥5 h·day-1) had less improvement in PaCO2 (mean difference (MD) -0.30 kPa, p<0.001 and -0.29 kPa, p<0.001, respectively) and HRQoL (standardised MD 0.10, p=0.002 and 0.11, p=0.02, respectively), but this effect did not persist to survival. PaCO2 improved more in patients with severe dyspnoea (MD -0.30, p=0.02), and HRQoL improved only in participants with fewer than three exacerbations (standardised MD 0.52, p=0.03). The results of the mediation analysis showed that the effect on HRQoL is mediated partially (23%) by a change in PaCO2.
Interpretation: With greater pressure support and better daily NIV usage, a larger improvement in PaCO2 and HRQoL is achieved. Importantly, we demonstrated that the beneficial effect of home NIV on HRQoL is only partially mediated through a reduction in diurnal PaCO2.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: T. Raveling reports a travel grant from Breas Medical. N.S. Hill reports consulting fees from Philips, consulting fees and payments from Fisher & Paykel, and participates in boards of Breas and Philips. C. Casanova reports consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Menarini and Novartis, and participates in boards of AstraZeneca and GlaxoSmithKline. E. Clini reports consulting fees from Chiesi Italia and Novartis, payments from AstraZeneca, Boehringer Ingelheim and GaxoSmithKline, and meeting/travel support from Boehringer Ingelheim and Chiesi. T. Köhnlein reports support from Grifols Deutschland GmbH. P.B. Murphy reports grants and payments from Fisher & Paykel, Resmed, Breas Medical and Philips Respironics, and payments from Chiesi and Genzyme. M.L. Duiverman reports grants from Resmed, Philips, Lowenstein, Vivisol, Sencure and Fisher & Paykel, and payments from Chiesi and Breas Medical. P.J. Wijkstra reports grants from Resmed, and grants and consulting fees from Philips.
Figures



References
-
- Macrea M, Oczkowski S, Rochwerg B, et al. Long-term noninvasive ventilation in chronic stable hypercapnic chronic obstructive pulmonary disease. An Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e74–e87. doi: 10.1164/rccm.202006-2382ST - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
