The many faces of COPD in real life: a longitudinal analysis of the NOVELTY cohort
- PMID: 38348246
- PMCID: PMC10860203
- DOI: 10.1183/23120541.00895-2023
The many faces of COPD in real life: a longitudinal analysis of the NOVELTY cohort
Abstract
Background: The diagnosis of COPD requires the demonstration of non-fully reversible airflow limitation by spirometry in the appropriate clinical context. Yet, there are patients with symptoms and relevant exposures suggestive of COPD with either normal spirometry (pre-COPD) or preserved ratio but impaired spirometry (PRISm). Their prevalence, clinical characteristics and associated outcomes in a real-life setting are unclear.
Methods: To investigate them, we studied 3183 patients diagnosed with COPD by their attending physician included in the NOVELTY study (clinicaltrials.gov identifier NCT02760329), a global, 3-year, observational, real-life cohort that included patients recruited from both primary and specialist care clinics in 18 countries.
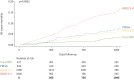
Results: We found that 1) approximately a quarter of patients diagnosed with (and treated for) COPD in real life did not fulfil the spirometric diagnostic criteria recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD), and could be instead categorised as pre-COPD (13%) or PRISm (14%); 2) disease burden (symptoms and exacerbations) was highest in GOLD 3-4 patients (exacerbations per person-year (PPY) 0.82) and lower but similar in those in GOLD 1-2, pre-COPD and PRISm (exacerbations range 0.27-0.43 PPY); 3) lung function decline was highest in pre-COPD and GOLD 1-2, and much less pronounced in PRISm and GOLD 3-4; 4) PRISm and pre-COPD were not stable diagnostic categories and change substantially over time; and 5) all-cause mortality was highest in GOLD 3-4, lowest in pre-COPD, and intermediate and similar in GOLD 1-2 and PRISm.
Conclusions: Patients diagnosed COPD in a real-life clinical setting present great diversity in symptom burden, progression and survival, warranting medical attention.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of Interest: A. Agustí reports support for the present manuscript as a member of the scientific committee of NOVELTY; and grants from GSK and AstraZeneca, consulting fees from GSK, AstraZeneca, Chiesi, Menarini and Sanofi, lecture honoraria from GSK, AstraZeneca, Chiesi, Menarini and Zambon, and an unpaid leadership role as chair of the board of directors of GOLD, outside the submitted work. Conflict of interest: R. Hughes is an employee of AstraZeneca. Conflict of interest: E. Rapsomaniki, L. Benton, S. Franzen and H. Mullerova report support for the present manuscript from AstraZeneca, and stock or stock options in AstraZeneca. Conflict of interest: B. Make reports support for the present manuscript from AstraZeneca; and grants from the NHLBI, the American Lung Association, the Department of Defense and AstraZeneca, royalties from Wolters Kluwer Health (Up-To-Date), consulting fees from AstraZeneca and Third Pole; lecture honoraria from AstraZeneca, Mt Sinai, Web MD, Novartis, the American College of Chest Physicians, Projects in Knowledge, the Eastern Pulmonary Society, Optimum Patient Care Global Limited, GlaxoSmithKline, Boston University Medical Center and Integritas Communications, advisory board participation with Spiration, GlaxoSmithKline, Mt Sinai, Boehringer Ingelheim, Mylan, Quintiles, the University of Wisconsin, Baystate Medical Center and AstraZeneca, outside the submitted work. Conflict of interest: R. del Olmo reports support for the present manuscript for medical writing from AstraZeneca; and reports lecture honoraria from AstraZeneca, GSK, Boehringer Ingelheim, Elea and Sanofi, and travel support from AstraZeneca, GSK and Boehringer Ingelheim, outside the submitted work. Conflict of interest: A. Papi reports support for the present manuscript from AstraZeneca; and grants from Chiesi, AstraZeneca, GSK, Sanofi and Agenzia Italiana Del Farmaco, consulting fees from Chiesi, AstraZeneca, GSK, Novartis, Sanofi, Avillion and Elpen Pharmaceutica, lecture honoraria from Chiesi, AstraZeneca, GSK, Menarini, Novartis, Zambon, Mundipharma, Sanofi, Edmond Pharma, Iqvia, Avillion and Elpen Pharmaceuticals, and advisory board participation with Chiesi, AstraZeneca, GSK, MSD, Novartis, Sanofi, Iqvia, Avillion and Elpen Pharmaceuticals, outside the submitted work. Conflict of interest: D. Price reports grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, the Respiratory Effectiveness Group, Sanofi Genzyme, Theravance and the UK National Health Service, consulting fees from Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance, WebMD Global LLC, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme; payment for expert testimony from GlaxoSmithKline; travel support from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis and Thermofisher, advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme and Thermofisher, stock or stock options from AKL Research and Development Ltd, Optimum Patient Care Ltd (Australia and the UK), the Observational and Pragmatic Research Institute Pte Ltd (Singapore) and Timestamp, and acts as peer reviewer for grant committees for UK Efficacy and the Mechanism Evaluation Programme and Health Technology Assessment, outside the submitted work. Conflict of interest: J. Vestbo reports consulting fees from AstraZeneca, ALK, Chiesi, GSK and Teva, and lecture honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi and GSK, outside the submitted work.
Figures




References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. 2023. https://goldcopd.org/2023-gold-report-2/
Associated data
LinkOut - more resources
Full Text Sources
Medical
