DNALI1 Promotes Neurodegeneration after Traumatic Brain Injury via Inhibition of Autophagosome-Lysosome Fusion
- PMID: 38348540
- PMCID: PMC11022701
- DOI: 10.1002/advs.202306399
DNALI1 Promotes Neurodegeneration after Traumatic Brain Injury via Inhibition of Autophagosome-Lysosome Fusion
Abstract
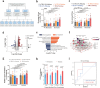
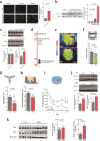
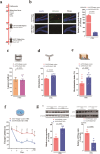
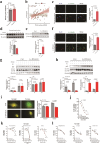
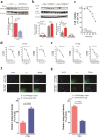
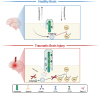
Traumatic brain injury (TBI) leads to progressive neurodegeneration that may be caused by chronic traumatic encephalopathy (CTE). However, the precise mechanism remains unclear. Herein, the study identifies a crucial protein, axonemal dynein light intermediate polypeptide 1 (DNALI1), and elucidated its potential pathogenic role in post-TBI neurodegeneration. The DNALI1 gene is systematically screened through analyses of Aging, Dementia, and TBI studies, confirming its elevated expression both in vitro and in vivo. Moreover, it is observed that altered DNALI1 expression under normal conditions has no discernible effect. However, upon overexpression, DNALI1 inhibits autophagosome-lysosome fusion, reduces autophagic flux, and exacerbates cell death under pathological conditions. DNALI1 silencing significantly enhances autophagic flux and alleviates neurodegeneration in a CTE model. These findings highlight DNALI1 as a potential key target for preventing TBI-related neurodegeneration.
Keywords: DNALI1; autophagy; chronic traumatic encephalopathy; neurodegeneration; traumatic brain injury.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia.J Neurol Neurosurg Psychiatry. 2019 Nov;90(11):1221-1233. doi: 10.1136/jnnp-2017-317557. Epub 2019 Sep 21. J Neurol Neurosurg Psychiatry. 2019. PMID: 31542723 Free PMC article. Review.
-
Multimodal Characterization of the Late Effects of Traumatic Brain Injury: A Methodological Overview of the Late Effects of Traumatic Brain Injury Project.J Neurotrauma. 2018 Jul 15;35(14):1604-1619. doi: 10.1089/neu.2017.5457. Epub 2018 May 3. J Neurotrauma. 2018. PMID: 29421973 Free PMC article.
-
Impaired autophagic flux is associated with the severity of trauma and the role of A2AR in brain cells after traumatic brain injury.Cell Death Dis. 2018 Feb 14;9(2):252. doi: 10.1038/s41419-018-0316-4. Cell Death Dis. 2018. PMID: 29449536 Free PMC article.
-
Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes.Toxicol Lett. 2020 Mar 15;321:32-43. doi: 10.1016/j.toxlet.2019.12.019. Epub 2019 Dec 17. Toxicol Lett. 2020. PMID: 31862506
-
Traumatic brain injury (TBI) in collision sports: Possible mechanisms of transformation into chronic traumatic encephalopathy (CTE).Metabolism. 2019 Nov;100S:153943. doi: 10.1016/j.metabol.2019.07.007. Metabolism. 2019. PMID: 31610856 Review.
Cited by
-
Recent advances in self-targeting natural product-based nanomedicines.J Nanobiotechnology. 2025 Jan 20;23(1):31. doi: 10.1186/s12951-025-03092-9. J Nanobiotechnology. 2025. PMID: 39833846 Free PMC article. Review.
-
Protein kinases in neurodegenerative diseases: current understandings and implications for drug discovery.Signal Transduct Target Ther. 2025 May 7;10(1):146. doi: 10.1038/s41392-025-02179-x. Signal Transduct Target Ther. 2025. PMID: 40328798 Free PMC article. Review.
-
Animal models of Alzheimer's disease: Current strategies and new directions.Zool Res. 2024 Nov 18;45(6):1385-1407. doi: 10.24272/j.issn.2095-8137.2024.274. Zool Res. 2024. PMID: 39572020 Free PMC article. Review.
-
Retinal Autophagy for Sustaining Retinal Integrity as a Proof of Concept for Age-Related Macular Degeneration.Int J Mol Sci. 2025 Jun 16;26(12):5773. doi: 10.3390/ijms26125773. Int J Mol Sci. 2025. PMID: 40565235 Free PMC article. Review.
-
Ferroptosis in ischemic stroke: Animal models and mechanisms.Zool Res. 2024 Nov 18;45(6):1235-1248. doi: 10.24272/j.issn.2095-8137.2024.239. Zool Res. 2024. PMID: 39397243 Free PMC article. Review.
References
-
- Blennow K., Brody D. L., Kochanek P. M., Levin H., McKee A., Ribbers G. M., Yaffe K., Zetterberg H., Nat. Rev. Dis. Primers 2016, 2, 16084. - PubMed
-
- a) Iaccarino C., Carretta A., Nicolosi F., Morselli C., J Neurosurg Sci 2018, 62, 535; - PubMed
- b) N. C. f. Health , Centers for Disease Control and Prevention , 2021.
-
- Dewan M. C., Rattani A., Gupta S., Baticulon R. E., Hung Y. C., Punchak M., Agrawal A., Adeleye A. O., Shrime M. G., Rubiano A. M., Rosenfeld J. V., Park K. B., J Neurosurg 2018, 130, 1080. - PubMed
-
- Shin M. K., Vázquez‐Rosa E., Koh Y., Dhar M., Chaubey K., Cintrón‐Pérez C. J., Barker S., Miller E., Franke K., Noterman M. F., Seth D., Allen R. S., Motz C. T., Rao S. R., Skelton L. A., Pardue M. T., Fliesler S. J., Wang C., Tracy T. E., Gan L., Liebl D. J., Savarraj J. P. J., Torres G. L., Ahnstedt H., McCullough L. D., Kitagawa R. S., Choi H. A., Zhang P., Hou Y., Chiang C. W., et al., Cell 2021, 184, 2715. - PMC - PubMed
MeSH terms
Grants and funding
- 2021YFC2500100/National Key Research and Development Project of China
- 82071191/National Natural Science Foundation of China
- 82301362/National Natural Science Foundation of China
- Z2023LC005/National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University
- ZYYC23016/West China Hospital 1.3.5 project for disciplines of excellence
- 24SYSX0093/Key Research Projects of Sichuan Province
- 2022ZDZX0021/Major Science & Technology Program of Sichuan Province
- BK20230277/Natural Science Foundation of Jiangsu Province
- 23KJB180023/Natural Science Foundation of Jiangsu Higher Education Institutions of China
- SZM2023037/Suzhou Science and Technology Bureau project
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
