Optimal duration of oxaliplatin-based adjuvant chemotherapy in patients with different risk factors for stage II-III colon cancer: a meta-analysis
- PMID: 38349218
- PMCID: PMC11093490
- DOI: 10.1097/JS9.0000000000001175
Optimal duration of oxaliplatin-based adjuvant chemotherapy in patients with different risk factors for stage II-III colon cancer: a meta-analysis
Abstract
Background: The duration of oxaliplatin-based chemotherapy in high-risk stage II, low-risk stage III, and high-risk stage III colon cancer (CC) patients is controversial. To reduce the risk of adverse events (AEs) without compromising efficacy while improving chemotherapy compliance is crucial.
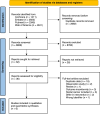
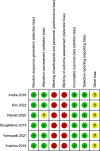
Methods: The authors searched Cochrane, Embase, Pubmed, and Web of Science databases for articles from inception to August 8, 2023, the main outcomes were disease-free survival, overall survival, chemotherapy completion rates, and AE frequency.
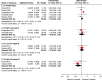
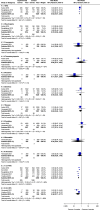
Results: Six randomized controlled trials (RCTs) involving 10 332 patients were included. Disease-free survival analysis revealed that only the high-risk stage III CC patients experienced better results with the 6-month FOLFOX regimen when compared with the 3-month regimen [Hazard ratio (HR): 1.32, 95% CI: 1.15-1.51, P <0.0001). Overall survival (OS) analysis revealed that extending the use of FOLFOX and CAPEOX regimens did not provide survival benefits for stage III CC patients (HR: 1.16, 95% CI: 0.9-1.49, and HR: 0.89, 95% CI: 0.67-1.18, P =0.40). The completion rate of the 3-month oxaliplatin-based adjuvant chemotherapy regimen was significantly higher than that of the 6-month regimen [Relative risk (RR): 1.16, 95% CI: 1.06-1.27, P =0.002]. Moreover, the 3-month regimen had significantly lower AE rates than the 6-month regimen (RR: 0.62, 95% CI: 0.57-0.68, P <0.00001), with differences mainly concentrated in grade 3/4 neutropenia (RR: 0.70, 95% CI: 0.59-0.85, P =0.0002), peripheral sensory neuropathy at ≥grade 2 (RR: 0.45, 95% CI: 0.38-0.53, P <0.00001), and hand-foot syndrome at ≥grade 2 (RR: 0.36, 95% CI: 0.17-0.77, P =0.009).
Conclusion: The 6-month FOLFOX regimen should only be recommended for high-risk stage III CC, while the 3-month regimen can be recommended for other stages. A 3-month CAPEOX regimen can be recommended for stage II-III CC.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures

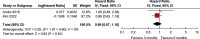

Similar articles
-
Assessment of Duration and Effects of 3 vs 6 Months of Adjuvant Chemotherapy in High-Risk Stage II Colorectal Cancer: A Subgroup Analysis of the TOSCA Randomized Clinical Trial.JAMA Oncol. 2020 Apr 1;6(4):547-551. doi: 10.1001/jamaoncol.2019.6486. JAMA Oncol. 2020. PMID: 32053133 Free PMC article. Clinical Trial.
-
Efficacy and Long-term Peripheral Sensory Neuropathy of 3 vs 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Colon Cancer: The ACHIEVE Phase 3 Randomized Clinical Trial.JAMA Oncol. 2019 Nov 1;5(11):1574-1581. doi: 10.1001/jamaoncol.2019.2572. JAMA Oncol. 2019. PMID: 31513248 Free PMC article. Clinical Trial.
-
Three- versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: the efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project.Ann Oncol. 2019 Aug 1;30(8):1304-1310. doi: 10.1093/annonc/mdz193. Ann Oncol. 2019. PMID: 31228203
-
Association Between Adjuvant Chemotherapy Duration and Survival Among Patients With Stage II and III Colon Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2019 May 3;2(5):e194154. doi: 10.1001/jamanetworkopen.2019.4154. JAMA Netw Open. 2019. PMID: 31099875 Free PMC article.
-
Adjuvant chemotherapy for high-risk stage II and stage III colon cancer: timing of initiation and optimal duration.J BUON. 2018 May-Jun;23(3):568-573. J BUON. 2018. PMID: 30003720
Cited by
-
Considerations regarding an updated meta-analysis of the optimal duration of oxaliplatin-based adjuvant chemotherapy in patients with different risk factors for stage II-III colon cancer.Int J Surg. 2024 Jul 1;110(7):4431-4432. doi: 10.1097/JS9.0000000000001350. Int J Surg. 2024. PMID: 39042074 Free PMC article. No abstract available.
-
Aberrant FAM135B attenuates the efficacy of chemotherapy in colorectal cancer by modulating SRSF1-mediated alternative splicing.Oncogene. 2024 Nov;43(48):3532-3544. doi: 10.1038/s41388-024-03189-9. Epub 2024 Oct 13. Oncogene. 2024. PMID: 39397154
References
-
- Wong MCS, Huang J, Lok V, et al. . Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol 2021;19:955–966.e61. - PubMed
-
- Leopa N, Dumitru E, Dumitru A, et al. . The clinicopathological differences of colon cancer in young adults versus older adults. J Adolesc Young Adult Oncol 2023;12:123–127. - PubMed
-
- Altieri MS, Thompson H, Pryor A, et al. . Incidence of colon resections is increasing in the younger populations: should an early initiation of colon cancer screening be implemented? Surg Endosc 2021;35:3636–3641. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

