Modelling costs of interventional pulmonary embolism treatment: implications of US trends for a European healthcare system
- PMID: 38349225
- PMCID: PMC11214584
- DOI: 10.1093/ehjacc/zuae019
Modelling costs of interventional pulmonary embolism treatment: implications of US trends for a European healthcare system
Abstract
Aims: Catheter-directed treatment (CDT) of acute pulmonary embolism (PE) is entering a growth phase in Europe following a steady increase in the USA in the past decade, but the potential economic impact on European healthcare systems remains unknown.
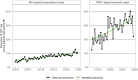
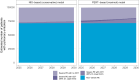
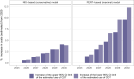
Methods and results: We built two statistical models for the monthly trend of proportion of CDT among patients with severe (intermediate- or high-risk) PE in the USA. The conservative model was based on admission data from the National Inpatient Sample (NIS) 2016-20 and the model reflecting increasing access to advanced treatment from the PERT™ national quality assurance database registry 2018-21. By applying these models to the forecast of annual PE-related hospitalizations in Germany, we calculated the annual number of severe PE cases and the expected increase in CDT use for the period 2025-30. The NIS-based model yielded a slow increase, reaching 3.1% (95% confidence interval 3.0-3.2%) among all hospitalizations with PE in 2030; in the PERT-based model, increase would be steeper, reaching 8.7% (8.3-9.2%). Based on current reimbursement rates, we estimated an increase of annual costs for PE-related hospitalizations in Germany ranging from 15.3 to 49.8 million euros by 2030. This calculation does not account for potential cost savings, including those from reduced length of hospital stay.
Conclusion: Our approach and results, which may be adapted to other European healthcare systems, provide a benchmark for healthcare costs expected to result from CDT. Data from ongoing trials on clinical benefits and cost savings are needed to determine cost-effectiveness and inform reimbursement decisions.
Keywords: Catheter-directed treatment; Cost of illness; Economic impact; Hospitalization costs; Pulmonary embolism.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: K.M, Kl.K., Ka.K., H.B., T.N., C.A., I.T.F., T.M.T., K.C., M.L., J.R., and L.V.: no disclosures; B.K.: AngioDynamics, Penumbra, Viz.ai, and Dexcom (consultant); R.P.R.: BMS and Janssen (institutional research support); Abbott, Dova, Inari, Janssen, and Penumbra (advisory/consultant); STORM-PE National Lead Investigator, Penumbra; The PERT Consortium™ President; J.M.M.: AngioDynamics, Penumbra, Argon Medical, Pavmed, Auxetics, Innova Vascular, Inquis Medical, Retriever Medical, and Boston Scientific (consultant); K.R.: Abbott Vascular, AngioDynamics, Auxetics, Becton Dickinson, Boston Scientific, Contego, Imperative Care/TRUVIC, Johnson and Johnson Biosense Webster, Medtronic, Neptune Medical, Philips, SurModics, and Terumo (consultant/advisory board); Access Vascular, Aerami, Althea Medical, Auxetics, Contego, Endospan, Imperative Care/TRUVIC, Innova Vascular, InspireMD, JanaCare, Magneto, MedAlliance, Neptune Medical, Orchestra, ProSomnus, Sealonix, Shockwave, Skydance, Summa Therapeutics, Thrombolex, Valcare, Vantis Vascular, Vasorum, and VuMedi (equity or stock options); NIH, Abiomed, Boston Scientific, Novo Nordisk, Penumbra, and Gettinge-Atrium (research grants via institution); The National PERT Consortium™, Board of Directors; S.B. Boston Scientific, Medtronic, Bayer, and Sanofi (institutional research support by Board); Boston Scientific, Penumbra, and Viatris (personal fees/honoraria); R.N.C.: Penumbra, Steering Committee; J.S.G.: advisor and research fees to the institution from Boston Scientific and Inari Medical; equity in Endovascular Engineering; R.A.L.: Boston Scientific and Medtronic (advisory board); Penumbra, Abbott Vascular, Neptune Medical, Bard Vascular, Cordis, Biosense Webster, Becton Dickinson, SurModics, and Abbott Vascular (speakers bureau); Philips Healthcare, Spectranetics, Terumo, Boston Scientific, Inari, Penumbra, Ethicon, Vesper, and Black Swan (research support); Imperative Vascular, Summa Vascular, Innova Vascular, and Thrombolex (equity shareholder); L.H.: MSD and Janssen (personal lecture/consultant fees); S.V.K.: Bayer AG, Boston Scientific, Daiichi-Sankyo, LumiraDx, and Penumbra (personal lecture/advisory fees and research grants to institution); MSD, Pfizer, and Bristol-Myers Squibb (personal lecture/advisory fees); E.A.S.: NIH/NHLBI K23HL150290, Food & Drug Administration, and SCAI (funding); Abbott, BD, Boston Scientific, Cook, Medtronic, and Philips (grants to institution); Abbott, BD, Boston Scientific, Cagent, Conavi, Cook, Cordis, InfraRedx, Medtronic, Philips, Recor, Shockwave, and VentureMed (speaking/consulting).
Figures
Similar articles
-
Drivers and recent trends of hospitalisation costs related to acute pulmonary embolism.Clin Res Cardiol. 2024 Apr 2. doi: 10.1007/s00392-024-02437-y. Online ahead of print. Clin Res Cardiol. 2024. PMID: 38565711
-
Inpatient resource use and cost burden of deep vein thrombosis and pulmonary embolism in the United States.Clin Ther. 2015 Jan 1;37(1):62-70. doi: 10.1016/j.clinthera.2014.10.024. Epub 2014 Dec 15. Clin Ther. 2015. PMID: 25524389
-
The implementation of a pulmonary embolism response team in the management of intermediate- or high-risk pulmonary embolism.J Vasc Surg Venous Lymphat Disord. 2019 Jul;7(4):493-500. doi: 10.1016/j.jvsv.2018.11.014. Epub 2019 Mar 29. J Vasc Surg Venous Lymphat Disord. 2019. PMID: 30930079
-
Healthcare resource utilisation and associated costs after low-risk pulmonary embolism: pre-specified analysis of the Home Treatment of Pulmonary Embolism (HoT-PE) study.Clin Res Cardiol. 2025 Feb;114(2):168-176. doi: 10.1007/s00392-023-02355-5. Epub 2024 Jan 3. Clin Res Cardiol. 2025. PMID: 38170252 Free PMC article.
-
Endovascular Management of Massive and Submassive Acute Pulmonary Embolism: Current Trends in Risk Stratification and Catheter-Directed Therapies.Curr Cardiol Rep. 2017 Jun;19(6):54. doi: 10.1007/s11886-017-0864-8. Curr Cardiol Rep. 2017. PMID: 28466280 Review.
Cited by
-
Time trends of catheter-directed treatment in acute pulmonary embolism in Germany.Res Pract Thromb Haemost. 2024 Dec 9;9(1):102651. doi: 10.1016/j.rpth.2024.102651. eCollection 2025 Jan. Res Pract Thromb Haemost. 2024. PMID: 39834528 Free PMC article.
-
Effective Management of Acute Pulmonary Embolism and Deep Vein Thrombosis: Insights From a Case Series on Procedure Benefits.Cureus. 2024 Oct 30;16(10):e72694. doi: 10.7759/cureus.72694. eCollection 2024 Oct. Cureus. 2024. PMID: 39618574 Free PMC article.
-
Intensive care treatment in acute pulmonary embolism in Germany, 2016 to 2020: a nationwide inpatient database study.Res Pract Thromb Haemost. 2024 Aug 8;8(6):102545. doi: 10.1016/j.rpth.2024.102545. eCollection 2024 Aug. Res Pract Thromb Haemost. 2024. PMID: 39318771 Free PMC article.
-
Drivers and recent trends of hospitalisation costs related to acute pulmonary embolism.Clin Res Cardiol. 2024 Apr 2. doi: 10.1007/s00392-024-02437-y. Online ahead of print. Clin Res Cardiol. 2024. PMID: 38565711
-
Pulmonary Embolism and Right Ventricular Dysfunction: Mechanism and Management.Cureus. 2024 Sep 30;16(9):e70561. doi: 10.7759/cureus.70561. eCollection 2024 Sep. Cureus. 2024. PMID: 39355468 Free PMC article. Review.
References
-
- Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation 2019;140:e774-e801. - PubMed
-
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. - PubMed
-
- Abumoawad A, Shatla I, Behrooz L, Eberhardt RT, Hamburg N, Sedhom R, et al. Temporal trends in the utilization of advanced therapies among patients with acute pulmonary embolism: insights from a national database. Eur Heart J Acute Cardiovasc Care 2023;12:711–713. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

