The Role of Anions in Rare-earth Activated Inorganic Host Materials for its Luminescence Characteristics
- PMID: 38349484
- PMCID: PMC11968488
- DOI: 10.1007/s10895-023-03561-0
The Role of Anions in Rare-earth Activated Inorganic Host Materials for its Luminescence Characteristics
Abstract
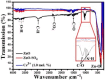
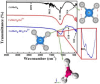
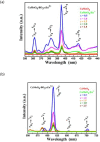
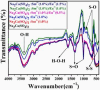
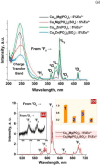
This work is inspired from the comprehensive work done by our research team aimed at improving the efficiency of white light emitting diodes (LEDs) through improvements in the colour rendering index of the red light (CRI), one of the primary colours of white light. Such work is triggered through the incorporation of anions (BO33-, PO43-, SO42-), either individually or as an integral part of dopant activated inorganic phosphor host materials. Numerous host materials such as ZnO, Y2O3, Ca3(PO4)2, CaMoO4, ABPO4, ABSO4 (where A represents alkali metals and B alkaline earth metals) have been considered ideal hosts materials for studying luminescence properties of materials (including other phosphors). In addition, red emitting dopants such as Sm3+, Eu3+ and Ce3+ have been incorporated into these host materials to achieve a higher CRI of red colour, an essential component of white light. The role anions in various materials is multifaceted; firstly, it acts as sensitizer whereby it absorbs excitation energy and transfers it non-radiatively to the dopants, secondly, it acts as a charge compensator to dopants with a charge of + 3, thirdly, it creates crystal fields that affects the electronic transitions of the dopants and fourthly, it creates a stable crystal structure that allows for dopant embedding. By understanding the exact role of these anions and their interactions with the host lattice and dopant ions, we could further optimize the luminescent properties of these activated host materials, which leads to higher efficiencies and performances in white light-emitting diodes and other lighting technologies. This work is a comprehensive review of the work undertaken by our research team aimed at enhancing the luminescent properties of WLEDs.
Keywords: Dopants; Energy transfer; Luminescence; Phosphor; WLEDs.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: N/A. Competing Interests: The authors declare no competing interests.
Figures



























References
-
- Xiao F, Xue YN, Ma YY, Zhang QY (2010) Ba2Ca(B3O6)2:Eu2+, Mn2+: A potential tunable blue-white-red phosphor for white light-emitting diodes. Phys B 405(3):891–895
-
- Hu SH, Lin YS, Su SH, Dai H, He JS (2022) Study on the enhancement of light intensity and high color rendering index of a white light emitting diode. J Electron Mater 52:270–275
-
- Balakrishna A, Swart HC, Ramaraghavulu R, Bedyal AK, Kroon RE, Ntwaeaborwa OM (2017) Structural evolution induced by substitution of designated molybdate sites (MoO42-) with different anionic groups (BO33-, PO43- and SO42-) in CaMoO4:Sm3+ phosphors – A study on color tunable luminescent properties. J Alloy Compd 727:224–237
-
- Caisheng X, Shikao S, Huili G, Ji Z (2010) Preparation and luminescent properties of Na5Eu(MoO4)4–x(PO4)x red phosphors for white-emitting diodes application. Mater Sci Forum 654–656:2025–2028
-
- Yan Z, Shikao S, Jing G, Ji Z (2010) Effects of SO42- or SiO32- doping on the photoluminescence of NaEu(MoO4)2 nanophosphors for light-emitting diodes. J Nanosci Nanotechnol 10(3):2156–3160 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Medical

