High-Reliability and Self-Rectifying Alkali Ion Memristor through Bottom Electrode Design and Dopant Incorporation
- PMID: 38349619
- PMCID: PMC10906085
- DOI: 10.1021/acsnano.3c11325
High-Reliability and Self-Rectifying Alkali Ion Memristor through Bottom Electrode Design and Dopant Incorporation
Abstract
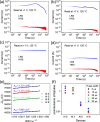
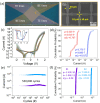
Ionic memristor devices are crucial for efficient artificial neural network computations in neuromorphic hardware. They excel in multi-bit implementation but face challenges like device reliability and sneak currents in crossbar array architecture (CAA). Interface-type ionic memristors offer low variation, self-rectification, and no forming process, making them suitable for CAA. However, they suffer from slow weight updates and poor retention and endurance. To address these issues, the study demonstrated an alkali ion self-rectifying memristor with an alkali metal reservoir formed by a bottom electrode design. By adopting Li metal as the adhesion layer of the bottom electrode, an alkali ion reservoir was formed at the bottom of the memristor layer by diffusion occurring during the atomic layer deposition process for the Na:TiO2 memristor layer. In addition, Al dopant was used to improve the retention characteristics by suppressing the diffusion of alkali cations. In the memristor device with optimized Al doping, retention characteristics of more than 20 h at 125 °C, endurance characteristics of more than 5.5 × 105, and high linearity/symmetry of weight update characteristics were achieved. In reliability tests on 100 randomly selected devices from a 32 × 32 CAA device, device-to-device and cycle-to-cycle variations showed low variation values within 81% and 8%, respectively.
Keywords: adhesion layer; alkali cation; artificial neural networks; reservoir layer; synaptic response.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Study on the Sodium-Doped Titania Interface-Type Memristor.ACS Appl Mater Interfaces. 2024 Apr 3;16(13):16453-16461. doi: 10.1021/acsami.3c19531. Epub 2024 Mar 22. ACS Appl Mater Interfaces. 2024. PMID: 38516695
-
Filamentary and Interface-Type Memristors Based on Tantalum Oxide for Energy-Efficient Neuromorphic Hardware.ACS Appl Mater Interfaces. 2022 Oct 5;14(39):44561-44571. doi: 10.1021/acsami.2c12296. Epub 2022 Sep 26. ACS Appl Mater Interfaces. 2022. PMID: 36164762
-
Sodium-Doped Titania Self-Rectifying Memristors for Crossbar Array Neuromorphic Architectures.Adv Mater. 2022 Feb;34(6):e2106913. doi: 10.1002/adma.202106913. Epub 2021 Dec 23. Adv Mater. 2022. PMID: 34773720
-
Research progress on solutions to the sneak path issue in memristor crossbar arrays.Nanoscale Adv. 2020 Mar 11;2(5):1811-1827. doi: 10.1039/d0na00100g. eCollection 2020 May 19. Nanoscale Adv. 2020. PMID: 36132530 Free PMC article. Review.
-
Self-Rectifying Memristors for Three-Dimensional In-Memory Computing.Adv Mater. 2024 Jan;36(4):e2307218. doi: 10.1002/adma.202307218. Epub 2023 Nov 27. Adv Mater. 2024. PMID: 37972344 Review.
Cited by
-
Memristive Ion Dynamics to Enable Biorealistic Computing.Chem Rev. 2025 Jan 22;125(2):745-785. doi: 10.1021/acs.chemrev.4c00587. Epub 2024 Dec 27. Chem Rev. 2025. PMID: 39729346 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

