Supporting Life Adjustment in Patients With Lung Cancer Through a Comprehensive Care Program: Protocol for a Controlled Before-and-After Trial
- PMID: 38349712
- PMCID: PMC10900087
- DOI: 10.2196/54707
Supporting Life Adjustment in Patients With Lung Cancer Through a Comprehensive Care Program: Protocol for a Controlled Before-and-After Trial
Abstract
Background: Lung cancer diagnosis affects an individual's quality of life as well as physical and emotional functioning. Information on survivorship care tends to be introduced at the end of treatment, but early intervention may affect posttreatment adjustment. However, to the best of our knowledge, no study has explored the effect of early information intervention on the return to work, family, and societal roles of lung cancer survivors.
Objective: We report the study protocol of a comprehensive care prehabilitation intervention designed to facilitate lung cancer survivors' psychological adjustment after treatment.
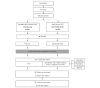
Methods: A comprehensive care program was developed based on a literature review and a qualitative study of patients with lung cancer and health professionals. The Lung Cancer Comprehensive Care Program consists of educational videos and follow-up visits by a family medicine physician. To prevent contamination, the control group received routine education, whereas the intervention group received routine care and intervention. Both groups completed questionnaires before surgery (T0) and at 1-month (T1), 6-month (T2), and 1-year (T3) follow-up visits after surgery. The primary outcome was survivors' psychological adjustment to cancer 6 months after pulmonary resection.
Results: The historical control group (n=441) was recruited from September 8, 2021, to April 20, 2022, and the intervention group (n=350) was recruited from April 22, 2022, to October 17, 2022. All statistical analyses will be performed upon completion of the study.
Conclusions: This study examined the effectiveness of an intervention that provided general and tailored informational support to lung cancer survivors, ranging from before to the end of treatment.
Trial registration: ClinicalTrials.gov NCT05078918; https://clinicaltrials.gov/ct2/show/NCT05078918.
International registered report identifier (irrid): DERR1-10.2196/54707.
Keywords: adjustment to cancer; cancer; comprehensive care; early intervention; education; educational; lung; lung cancer; lungs; oncology; prehabilitation; pulmonary; respiratory; return to work; survivor; survivors; survivorship; unmet needs.
©Wonyoung Jung, Alice Ahn, Genehee Lee, Sunga Kong, Danbee Kang, Dongok Lee, Tae Eun Kim, Young Mog Shim, Hong Kwan Kim, Jongho Cho, Juhee Cho, Dong Wook Shin. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 13.02.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
A Smartphone App-Based Mindfulness Intervention for Cancer Survivors: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2020 May 11;9(5):e15178. doi: 10.2196/15178. JMIR Res Protoc. 2020. PMID: 32390591 Free PMC article.
-
The Finding My Way UK Clinical Trial: Adaptation Report and Protocol for a Replication Randomized Controlled Efficacy Trial of a Web-Based Psychological Program to Support Cancer Survivors.JMIR Res Protoc. 2021 Sep 20;10(9):e31976. doi: 10.2196/31976. JMIR Res Protoc. 2021. PMID: 34542420 Free PMC article.
-
Internet-Delivered Cognitive Behavioral Treatment for Chronic Pain in Adolescent Survivors of Childhood Cancer: Protocol for a Single-Group Feasibility Trial.JMIR Res Protoc. 2023 Aug 1;12:e45804. doi: 10.2196/45804. JMIR Res Protoc. 2023. PMID: 37526959 Free PMC article.
-
Exploring Interactive Survivorship Care Plans to Support Breast Cancer Survivors: Protocol for a Randomized Controlled Trial.JMIR Res Protoc. 2020 Dec 4;9(12):e23414. doi: 10.2196/23414. JMIR Res Protoc. 2020. PMID: 33274725 Free PMC article.
-
Behavioural modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation.Health Technol Assess. 2020 Sep;24(46):1-490. doi: 10.3310/hta24460. Health Technol Assess. 2020. PMID: 32975190 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. https://onlinelibrary.wiley.com/doi/10.3322/caac.21660 - DOI - DOI - PubMed
-
- Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG. Prediction of cancer incidence and mortality in Korea, 2021. Cancer Res Treat. 2021;53(2):316–322. doi: 10.4143/crt.2021.290. https://europepmc.org/abstract/MED/33735558 crt.2021.290 - DOI - PMC - PubMed
-
- Shin DW, Cho JH, Yoo JE, Cho J, Yoon DW, Lee G, Shin S, Kim HK, Choi YS, Kim J, Zo JI, Shim YM. Conditional survival of surgically treated patients with lung cancer: a comprehensive analyses of overall, recurrence-free, and relative survival. Cancer Res Treat. 2021;53(4):1057–1071. doi: 10.4143/crt.2020.1308. https://europepmc.org/abstract/MED/33705624 crt.2020.1308 - DOI - PMC - PubMed
-
- Yang P, Cheville AL, Wampfler JA, Garces YI, Jatoi A, Clark MM, Cassivi SD, Midthun DE, Marks RS, Aubry MC, Okuno SH, Williams BA, Nichols FC, Trastek VF, Sugimura H, Sarna L, Allen MS, Deschamps C, Sloan JA. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol. 2012;7(1):64–70. doi: 10.1097/JTO.0b013e3182397b3e. https://linkinghub.elsevier.com/retrieve/pii/S1556-0864(15)31760-3 S1556-0864(15)31760-3 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

