Connexin43 in mesenchymal lineage cells regulates body adiposity and energy metabolism in mice
- PMID: 38349739
- PMCID: PMC11063945
- DOI: 10.1172/jci.insight.170016
Connexin43 in mesenchymal lineage cells regulates body adiposity and energy metabolism in mice
Abstract
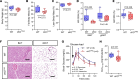
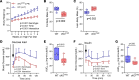
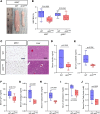
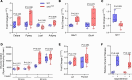
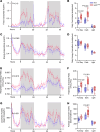
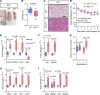
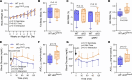
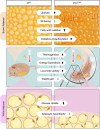
Connexin43 (Cx43) is the most abundant gap junction protein present in the mesenchymal lineage. In mature adipocytes, Cx43 mediates white adipose tissue (WAT) beiging in response to cold exposure and maintains the mitochondrial integrity of brown adipose tissue (BAT). We found that genetic deletion of Gja1 (Cx43 gene) in cells that give rise to chondro-osteogenic and adipogenic precursors driven by the Dermo1/Twist2 promoter led to lower body adiposity and partial protection against the weight gain and metabolic syndrome induced by a high-fat diet (HFD) in both sexes. These protective effects were related to increased locomotion, fuel utilization, energy expenditure, nonshivering thermogenesis, and better glucose tolerance in conditionally Gja1-ablated mice. Accordingly, Gja1-mutant mice exhibited reduced adipocyte hypertrophy, partially preserved insulin sensitivity, increased BAT lipolysis, and decreased whitening under HFD. This metabolic phenotype was not reproduced with more restricted Gja1 ablation in differentiated adipocytes, suggesting that Cx43 in adipocyte progenitors or other targeted cells restrains energy expenditures and promotes fat accumulation. These results reveal what we believe is a hitherto unknown action of Cx43 in adiposity, and offer a promising new pharmacologic target for improving metabolic balance in diabetes and obesity.
Keywords: Adipose tissue; Adult stem cells; Metabolism; Obesity.
Conflict of interest statement
Figures

Update of
-
Connexin43 in mesenchymal lineage cells regulates body adiposity and energy metabolism in mice.bioRxiv [Preprint]. 2024 Jan 7:2024.01.05.574415. doi: 10.1101/2024.01.05.574415. bioRxiv. 2024. Update in: JCI Insight. 2024 Feb 13;9(6):e170016. doi: 10.1172/jci.insight.170016. PMID: 38260624 Free PMC article. Updated. Preprint.
Similar articles
-
Connexin43 in mesenchymal lineage cells regulates body adiposity and energy metabolism in mice.bioRxiv [Preprint]. 2024 Jan 7:2024.01.05.574415. doi: 10.1101/2024.01.05.574415. bioRxiv. 2024. Update in: JCI Insight. 2024 Feb 13;9(6):e170016. doi: 10.1172/jci.insight.170016. PMID: 38260624 Free PMC article. Updated. Preprint.
-
Connexin 43 is required for the maintenance of mitochondrial integrity in brown adipose tissue.Sci Rep. 2017 Aug 2;7(1):7159. doi: 10.1038/s41598-017-07658-y. Sci Rep. 2017. PMID: 28769076 Free PMC article.
-
AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue.Front Physiol. 2018 Feb 21;9:122. doi: 10.3389/fphys.2018.00122. eCollection 2018. Front Physiol. 2018. PMID: 29515462 Free PMC article.
-
Adipocyte lineages: tracing back the origins of fat.Biochim Biophys Acta. 2014 Mar;1842(3):340-51. doi: 10.1016/j.bbadis.2013.05.027. Epub 2013 Jun 4. Biochim Biophys Acta. 2014. PMID: 23747579 Free PMC article. Review.
-
Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans.Horm Mol Biol Clin Investig. 2017 Jun 23;31(2):/j/hmbci.2017.31.issue-2/hmbci-2017-0016/hmbci-2017-0016.xml. doi: 10.1515/hmbci-2017-0016. Horm Mol Biol Clin Investig. 2017. PMID: 28672737 Review.
Cited by
-
Exploring the Potent Roles of an Internally Translated Truncated Connexin-43 Isoform.Biology (Basel). 2024 Dec 13;13(12):1046. doi: 10.3390/biology13121046. Biology (Basel). 2024. PMID: 39765713 Free PMC article. Review.
-
Cold Exposure Rejuvenates the Metabolic Phenotype of Panx1-/- Mice.Biomolecules. 2024 Aug 25;14(9):1058. doi: 10.3390/biom14091058. Biomolecules. 2024. PMID: 39334824 Free PMC article.
-
Brown adipose tissue: a potential therapeutic target for preventing cardiovascular disease in metabolic disorders.Diabetol Metab Syndr. 2025 Aug 2;17(1):311. doi: 10.1186/s13098-025-01892-5. Diabetol Metab Syndr. 2025. PMID: 40753433 Free PMC article.
-
Repetitive transcranial magnetic stimulation promotes motor function recovery in mice after spinal cord injury via regulation of the Cx43-autophagy loop.J Orthop Surg Res. 2024 Jul 2;19(1):387. doi: 10.1186/s13018-024-04879-6. J Orthop Surg Res. 2024. PMID: 38956661 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

