A mutation in F-actin polymerization factor suppresses the distal arthrogryposis type 5 PIEZO2 pathogenic variant in Caenorhabditis elegans
- PMID: 38349741
- PMCID: PMC10911111
- DOI: 10.1242/dev.202214
A mutation in F-actin polymerization factor suppresses the distal arthrogryposis type 5 PIEZO2 pathogenic variant in Caenorhabditis elegans
Abstract
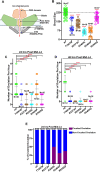
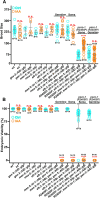
The mechanosensitive PIEZO channel family has been linked to over 26 disorders and diseases. Although progress has been made in understanding these channels at the structural and functional levels, the underlying mechanisms of PIEZO-associated diseases remain elusive. In this study, we engineered four PIEZO-based disease models using CRISPR/Cas9 gene editing. We performed an unbiased chemical mutagen-based genetic suppressor screen to identify putative suppressors of a conserved gain-of-function variant pezo-1[R2405P] that in human PIEZO2 causes distal arthrogryposis type 5 (DA5; p. R2718P). Electrophysiological analyses indicate that pezo-1(R2405P) is a gain-of-function allele. Using genomic mapping and whole-genome sequencing approaches, we identified a candidate suppressor allele in the C. elegans gene gex-3. This gene is an ortholog of human NCKAP1 (NCK-associated protein 1), a subunit of the Wiskott-Aldrich syndrome protein (WASP)-verprolin homologous protein (WAVE/SCAR) complex, which regulates F-actin polymerization. Depletion of gex-3 by RNAi, or with the suppressor allele gex-3(av259[L353F]), significantly increased brood size and ovulation rate, as well as alleviating the crushed oocyte phenotype of the pezo-1(R2405P) mutant. Expression of GEX-3 in the soma is required to rescue the brood size defects in pezo-1(R2405P) animals. Actin organization and orientation were disrupted and distorted in the pezo-1 mutants. Mutation of gex-3(L353F) partially alleviated these defects. The identification of gex-3 as a suppressor of the pathogenic variant pezo-1(R2405P) suggests that the PIEZO coordinates with the cytoskeleton regulator to maintain the F-actin network and provides insight into the molecular mechanisms of DA5 and other PIEZO-associated diseases.
Keywords: Disease modeling in C. elegans; Distal arthrogryposis type 5; Forward genetic screening; Genetic suppressor; PIEZO channel; WAVE complex GEX-3.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures







Update of
-
Mutation in F-actin Polymerization Factor Suppresses Distal Arthrogryposis Type 5 (DA5) PIEZO2 Pathogenic Variant in Caenorhabditis elegans.bioRxiv [Preprint]. 2023 Jul 24:2023.07.24.550416. doi: 10.1101/2023.07.24.550416. bioRxiv. 2023. Update in: Development. 2024 Feb 15;151(4):dev202214. doi: 10.1242/dev.202214. PMID: 37546771 Free PMC article. Updated. Preprint.
References
-
- Albuisson, J., Murthy, S. E., Bandell, M., Coste, B., Louis-Dit-Picard, H., Mathur, J., Fénéant-Thibault, M., Tertian, G., de Jaureguiberry, J.-P., Syfuss, P.-Y.et al. (2013). Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat. Commun. 4, 1884. 10.1038/ncomms2899 - DOI - PMC - PubMed
-
- Andolfo, I., Alper, S. L., De Franceschi, L., Auriemma, C., Russo, R., De Falco, L., Vallefuoco, F., Esposito, M. R., Vandorpe, D. H., Shmukler, B. E.et al. (2013). Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121, 3925-3935, S3921–3912. 10.1182/blood-2013-02-482489 - DOI - PubMed
-
- Ashley, G. E., Duong, T., Levenson, M. T., Martinez, M. A. Q., Johnson, L. C., Hibshman, J. D., Saeger, H. N., Palmisano, N. J., Doonan, R., Martinez-Mendez, R.et al. (2021). An expanded auxin-inducible degron toolkit for Caenorhabditis elegans. Genetics 217, iyab006. 10.1093/genetics/iyab006 - DOI - PMC - PubMed
-
- Atcha, H., Jairaman, A., Holt, J. R., Meli, V. S., Nagalla, R. R., Veerasubramanian, P. K., Brumm, K. T., Lim, H. E., Othy, S., Cahalan, M. D.et al. (2021). Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 12, 3256. 10.1038/s41467-021-23482-5 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

