Corticosteroids impair epithelial regeneration in immune-mediated intestinal damage
- PMID: 38349762
- PMCID: PMC10977993
- DOI: 10.1172/JCI155880
Corticosteroids impair epithelial regeneration in immune-mediated intestinal damage
Abstract
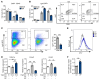
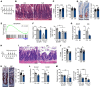
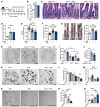
Corticosteroid treatment (CST) failure is associated with poor outcomes for patients with gastrointestinal (GI) graft-versus-host disease (GVHD). CST is intended to target the immune system, but the glucocorticoid receptor (GR) is widely expressed, including within the intestines, where its effects are poorly understood. Here, we report that corticosteroids (CS) directly targeted intestinal epithelium, potentially worsening immune-mediated GI damage. CS administered to mice in vivo and intestinal organoid cultures ex vivo reduced epithelial proliferation. Following irradiation, immediate CST mitigated GI damage but delayed treatment attenuated regeneration and exacerbated damage. In a murine steroid-refractory (SR) GVHD model, CST impaired epithelial regeneration, worsened crypt loss, and reduced intestinal stem cell (ISC) frequencies. CST also exacerbated immune-mediated damage in organoid cultures with SR, GR-deficient T cells or IFN-γ. These findings correlated with CS-dependent changes in apoptosis-related gene expression and STAT3-related epithelial proliferation. Conversely, IL-22 administration enhanced STAT3 activity and overcame CS-mediated attenuation of regeneration, reducing crypt loss and promoting ISC expansion in steroid-treated mice with GVHD. Therefore, CST has the potential to exacerbate GI damage if it fails to control the damage-inducing immune response, but this risk may be countered by strategies augmenting epithelial regeneration, thus providing a rationale for clinical approaches combining such tissue-targeted therapies with immunosuppression.
Keywords: Bone marrow transplantation; Immunology; Mouse models; Mouse stem cells; Transplantation.
Figures



Comment in
- The road to refractory graft-versus-host disease is paved with good intentions doi: 10.1172/JCI177728
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

