Improved Disease-Free Survival With Adjuvant Chemotherapy After Nephroureterectomy for Upper Tract Urothelial Cancer: Final Results of the POUT Trial
- PMID: 38350047
- PMCID: PMC11095877
- DOI: 10.1200/JCO.23.01659
Improved Disease-Free Survival With Adjuvant Chemotherapy After Nephroureterectomy for Upper Tract Urothelial Cancer: Final Results of the POUT Trial
Abstract
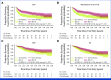
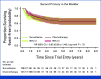
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.POUT was a phase III, randomized, open-label trial, including 261 patients with muscle-invasive or lymph node-positive, nonmetastatic upper tract urothelial cancer (UTUC) randomly assigned after radical nephroureterectomy to platinum-based chemotherapy (132) or surveillance (129). Primary outcome analysis demonstrated that chemotherapy improved disease-free survival (DFS). At that time, the planned secondary outcome analysis of overall survival (OS) was immature. By February 2022, 50 and 67 DFS events had occurred in the chemotherapy and surveillance groups, respectively, at a median follow-up of 65 months. The 5-year DFS was 62% versus 45%, univariable hazard ratio (HR), 0.55 (95% CI, 0.38 to 0.80, P = .001). The restricted mean survival time (RMST) was 18 months longer (95% CI, 6 to 30) in the chemotherapy arm. There were 46 and 60 deaths in the chemotherapy and control arms, respectively. The 5-year OS was 66% versus 57%, with univariable HR, 0.68 (95% CI, 0.46 to 1.00, P = .049) and RMST difference 11 months (95% CI, 1 to 21). Treatment effects were consistent across chemotherapy regimens (carboplatin or cisplatin) and disease stage. Toxicities were similar to those previously reported, and there were no clinically relevant differences in quality of life between arms. In summary, although OS was not the primary outcome measure, the updated results add further support for the use of adjuvant chemotherapy in patients with UTUC, suggesting long-term benefits.
Trial registration: ClinicalTrials.gov NCT01993979.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526.
-
- Rouprêt M, Seisen T, Birtle AJ, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur Urol. 2023;84:49–64. - PubMed
-
- Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2012;23:406–410. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

