Treatment-specific risk of subsequent malignant neoplasms in five-year survivors of diffuse large B-cell lymphoma
- PMID: 38350338
- PMCID: PMC10937196
- DOI: 10.1016/j.esmoop.2024.102248
Treatment-specific risk of subsequent malignant neoplasms in five-year survivors of diffuse large B-cell lymphoma
Abstract
Background: The introduction of rituximab significantly improved the prognosis of diffuse large B-cell lymphoma (DLBCL), emphasizing the importance of evaluating the long-term consequences of exposure to radiotherapy, alkylating agents and anthracycline-containing (immuno)chemotherapy among DLBCL survivors.
Methods: Long-term risk of subsequent malignant neoplasms (SMNs) was examined in a multicenter cohort comprising 2373 5-year DLBCL survivors treated at ages 15-61 years in 1989-2012. Observed SMN numbers were compared with expected cancer incidence to estimate standardized incidence ratios (SIRs) and absolute excess risks (AERs/10 000 person-years). Treatment-specific risks were assessed using multivariable Cox regression.
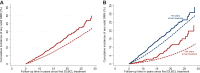
Results: After a median follow-up of 13.8 years, 321 survivors developed one or more SMNs (SIR 1.5, 95% CI 1.3-1.8, AER 51.8). SIRs remained increased for at least 20 years after first-line treatment (SIR ≥20-year follow-up 1.5, 95% CI 1.0-2.2, AER 81.8) and were highest among patients ≤40 years at first DLBCL treatment (SIR 2.7, 95% CI 2.0-3.5). Lung (SIR 2.0, 95% CI 1.5-2.7, AER 13.4) and gastrointestinal cancers (SIR 1.5, 95% CI 1.2-2.0, AER 11.8) accounted for the largest excess risks. Treatment with >4500 mg/m2 cyclophosphamide/>300 mg/m2 doxorubicin versus ≤2250 mg/m2/≤150 mg/m2, respectively, was associated with increased solid SMN risk (hazard ratio 1.5, 95% CI 1.0-2.2). Survivors who received rituximab had a lower risk of subdiaphragmatic solid SMNs (hazard ratio 0.5, 95% CI 0.3-1.0) compared with survivors who did not receive rituximab.
Conclusion: Five-year DLBCL survivors have an increased risk of SMNs. Risks were higher for survivors ≤40 years at first treatment and survivors treated with >4500 mg/m2 cyclophosphamide/>300 mg/m2 doxorubicin, and may be lower for survivors treated in the rituximab era, emphasizing the need for studies with longer follow-up for rituximab-treated patients.
Keywords: alkylating agents; anthracyclines; diffuse large B-cell lymphoma; radiotherapy; subsequent neoplasms; survivorship.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure MJK has received research support from Kite/Gilead and financial compensation for attending advisory boards and/or presentations from Roche, Kite/Gilead, Novartis, BMS/Celgene, Miltenyi Biotec, Takeda and Adicet Bio. All other authors have declared no conflicts of interest.
Figures

References
-
- McKelvey E.M., Gottlieb J.A., Wilson H.E., et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38(4):1484–1493. - PubMed
-
- Fisher R.I., Gaynor E.R., Dahlberg S., et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002–1006. - PubMed
-
- Coiffier B., Lepage E., Briere J., et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Pfreundschuh M., Kuhnt E., Trumper L., et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013–1022. - PubMed
-
- IKNL. Het diffuus grootcellig B-cellymfoom in Nederland, 2014-2016. Landelijk rapport van het hemato-oncologieregister van de Nederlandse Kankerregistratie; 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

