miR-1202 acts as anti-oncomiR in myeloid leukaemia by down-modulating GATA-1S expression
- PMID: 38350611
- PMCID: PMC10864098
- DOI: 10.1098/rsob.230319
miR-1202 acts as anti-oncomiR in myeloid leukaemia by down-modulating GATA-1S expression
Abstract
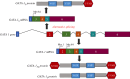
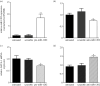
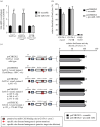
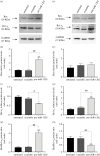
Transient abnormal myelopoiesis (TAM) is a Down syndrome-related pre-leukaemic condition characterized by somatic mutations in the haematopoietic transcription factor GATA-1 that result in exclusive production of its shorter isoform (GATA-1S). Given the common hallmark of altered miRNA expression profiles in haematological malignancies and the pro-leukaemic role of GATA-1S, we aimed to search for miRNAs potentially able to modulate the expression of GATA-1 isoforms. Starting from an in silico prediction of miRNA binding sites in the GATA-1 transcript, miR-1202 came into our sight as potential regulator of GATA-1 expression. Expression studies in K562 cells revealed that miR-1202 directly targets GATA-1, negatively regulates its expression, impairs GATA-1S production, reduces cell proliferation, and increases apoptosis sensitivity. Furthermore, data from TAM and myeloid leukaemia patients provided substantial support to our study by showing that miR-1202 down-modulation is accompanied by increased GATA-1 levels, with more marked effects on GATA-1S. These findings indicate that miR-1202 acts as an anti-oncomiR in myeloid cells and may impact leukaemogenesis at least in part by down-modulating GATA-1S levels.
Keywords: Down syndrome; GATA-1; alternative splicing; miR-1202; myeloid leukaemia.
Conflict of interest statement
We declare we have no competing interests.
Figures





Similar articles
-
GATA1 and cooperating mutations in myeloid leukaemia of Down syndrome.IUBMB Life. 2020 Jan;72(1):119-130. doi: 10.1002/iub.2197. Epub 2019 Nov 26. IUBMB Life. 2020. PMID: 31769932 Review.
-
Haematopoietic development and leukaemia in Down syndrome.Br J Haematol. 2014 Dec;167(5):587-99. doi: 10.1111/bjh.13096. Epub 2014 Aug 22. Br J Haematol. 2014. PMID: 25155832 Review.
-
PRAME immunohistochemical staining in transient abnormal myelopoiesis and myeloid leukemia associated with Down syndrome.Ann Clin Lab Sci. 2015 Spring;45(2):121-7. Ann Clin Lab Sci. 2015. PMID: 25887863
-
Exploring the Leukemogenic Potential of GATA-1S, the Shorter Isoform of GATA-1: Novel Insights into Mechanisms Hampering Respiratory Chain Complex II Activity and Limiting Oxidative Phosphorylation Efficiency.Antioxidants (Basel). 2021 Oct 12;10(10):1603. doi: 10.3390/antiox10101603. Antioxidants (Basel). 2021. PMID: 34679737 Free PMC article.
-
Recent advances in the understanding of transient abnormal myelopoiesis in Down syndrome.Pediatr Int. 2019 Mar;61(3):222-229. doi: 10.1111/ped.13776. Epub 2019 Mar 4. Pediatr Int. 2019. PMID: 30593694 Review.
Cited by
-
The Molecular Interplay Between p53-Mediated Ferroptosis and Non-Coding RNAs in Cancer.Int J Mol Sci. 2025 Jul 9;26(14):6588. doi: 10.3390/ijms26146588. Int J Mol Sci. 2025. PMID: 40724837 Free PMC article. Review.
References
-
- Halsey C, Docherty M, Mcneill M, Gilchrist D, Le Brocq M, Gibson B, Graham G. 2012. The GATA1s isoform is normally down-regulated during terminal haematopoietic differentiation and over-expression leads to failure to repress MYB, CCND2 and SKI during erythroid differentiation of K562 cells. J. Hematol. Oncol. 5, 45. (10.1186/1756-8722-5-45) - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

