Impact of the COVID-19 related border restrictions on influenza and other common respiratory viral infections in New Zealand
- PMID: 38350715
- PMCID: PMC10864123
- DOI: 10.1111/irv.13247
Impact of the COVID-19 related border restrictions on influenza and other common respiratory viral infections in New Zealand
Abstract
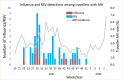
Background: New Zealand's (NZ) complete absence of community transmission of influenza and respiratory syncytial virus (RSV) after May 2020, likely due to COVID-19 elimination measures, provided a rare opportunity to assess the impact of border restrictions on common respiratory viral infections over the ensuing 2 years.
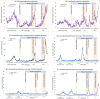
Methods: We collected the data from multiple surveillance systems, including hospital-based severe acute respiratory infection surveillance, SHIVERS-II, -III and -IV community cohorts for acute respiratory infection (ARI) surveillance, HealthStat sentinel general practice (GP) based influenza-like illness surveillance and SHIVERS-V sentinel GP-based ARI surveillance, SHIVERS-V traveller ARI surveillance and laboratory-based surveillance. We described the data on influenza, RSV and other respiratory viral infections in NZ before, during and after various stages of the COVID related border restrictions.
Results: We observed that border closure to most people, and mandatory government-managed isolation and quarantine on arrival for those allowed to enter, appeared to be effective in keeping influenza and RSV infections out of the NZ community. Border restrictions did not affect community transmission of other respiratory viruses such as rhinovirus and parainfluenza virus type-1. Partial border relaxations through quarantine-free travel with Australia and other countries were quickly followed by importation of RSV in 2021 and influenza in 2022.
Conclusion: Our findings inform future pandemic preparedness and strategies to model and manage the impact of influenza and other respiratory viral threats.
Keywords: acute respiratory illness; common respiratory viral infections; influenza infection; public health and social measures; respiratory syncytial viral infection; severe acute respiratory infections.
© 2024 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- New_Zealand_Government . New Zealand COVID‐19 alert levels summary. https://covid19.govt.nz/assets/resources/tables/COVID-19-alert-levels-su.... 2020; (4‐Sept‐2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

