Tretinoin improves the anti-cancer response to cyclophosphamide, in a model-selective manner
- PMID: 38350880
- PMCID: PMC10865642
- DOI: 10.1186/s12885-024-11915-5
Tretinoin improves the anti-cancer response to cyclophosphamide, in a model-selective manner
Abstract
Background: Chemotherapy is included in treatment regimens for many solid cancers, but when administered as a single agent it is rarely curative. The addition of immune checkpoint therapy to standard chemotherapy regimens has improved response rates and increased survival in some cancers. However, most patients do not respond to treatment and immune checkpoint therapy can cause severe side effects. Therefore, there is a need for alternative immunomodulatory drugs that enhance chemotherapy.
Methods: We used gene expression data from cyclophosphamide (CY) responders and non-responders to identify existing clinically approved drugs that could phenocopy a chemosensitive tumor microenvironment (TME), and tested combination treatments in multiple murine cancer models.
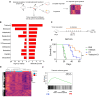
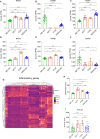
Results: The vitamin A derivative tretinoin was the top predicted upstream regulator of response to CY. Tretinoin pre-treatment induced an inflammatory, interferon-associated TME, with increased infiltration of CD8 + T cells, sensitizing the tumor to subsequent chemotherapy. However, while combination treatment significantly improved survival and cure rate in a CD4+ and CD8+ T cell dependent manner in AB1-HA murine mesothelioma, this effect was model-selective, and could not be replicated using other cell lines.
Conclusions: Despite the promising data in one model, the inability to validate the efficacy of combination treatment in multiple cancer models deprioritizes tretinoin/cyclophosphamide combination therapy for clinical translation.
Keywords: Combination treatment; Cyclophosphamide; Interferon; Sensitisation; Tretinoin.
© 2024. The Author(s).
Conflict of interest statement
W.J.L., R.A.L. and A.K.N. received research funding from Douglas Pharmaceuticals and all the other authors declare no competing interest.
Figures



Similar articles
-
Retinoic Acid Induces an IFN-Driven Inflammatory Tumour Microenvironment, Sensitizing to Immune Checkpoint Therapy.Front Oncol. 2022 Mar 24;12:849793. doi: 10.3389/fonc.2022.849793. eCollection 2022. Front Oncol. 2022. PMID: 35402250 Free PMC article.
-
Immunomodulatory properties of antineoplastic drugs administered in conjunction with GM-CSF-secreting cancer cell vaccines.Int J Oncol. 1998 Jan;12(1):161-70. doi: 10.3892/ijo.12.1.161. Int J Oncol. 1998. PMID: 9454900
-
Cyclophosphamide chemotherapy sensitizes tumor cells to TRAIL-dependent CD8 T cell-mediated immune attack resulting in suppression of tumor growth.PLoS One. 2009 Sep 10;4(9):e6982. doi: 10.1371/journal.pone.0006982. PLoS One. 2009. PMID: 19746156 Free PMC article.
-
Adoptive transfer of ex vivo-activated memory T-cell subsets with cyclophosphamide provides effective tumor-specific chemoimmunotherapy of advanced metastatic murine melanoma and carcinoma.Int J Cancer. 1995 May 16;61(4):580-6. doi: 10.1002/ijc.2910610424. Int J Cancer. 1995. PMID: 7759164
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

