In-situ formatting donor-acceptor polymer with giant dipole moment and ultrafast exciton separation
- PMID: 38350993
- PMCID: PMC10864376
- DOI: 10.1038/s41467-024-45604-5
In-situ formatting donor-acceptor polymer with giant dipole moment and ultrafast exciton separation
Abstract
Donor-acceptor semiconducting polymers present countless opportunities for application in photocatalysis. Previous studies have showcased their advantages through direct bottom-up methods. Unfortunately, these approaches often involve harsh reaction conditions, overlooking the impact of uncontrolled polymerization degrees on photocatalysis. Besides, the mechanism behind the separation of electron-hole pairs (excitons) in donor-acceptor polymers remains elusive. This study presents a post-synthetic method involving the light-induced transformation of the building blocks of hyper-cross-linked polymers from donor-carbon-donor to donor-carbon-acceptor states, resulting in a polymer with a substantial intramolecular dipole moment. Thus, excitons are efficiently separated in the transformed polymer. The utility of this strategy is exemplified by the enhanced photocatalytic hydrogen peroxide synthesis. Encouragingly, our observations reveal the formation of intramolecular charge transfer states using time-resolved techniques, confirming transient exciton behavior involving separation and relaxation. This light-induced method not only guides the development of highly efficient donor-acceptor polymer photocatalysts but also applies to various fields, including organic solar cells, light-emitting diodes, and sensors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

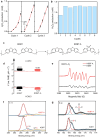

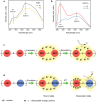
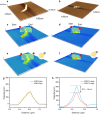


References
-
- Banerjee T, et al. Polymer photocatalysts for solar-to-chemical energy conversion. Nat. Rev. Mater. 2020;6:168–190. doi: 10.1038/s41578-020-00254-z. - DOI
Grants and funding
- 2022CFA001/Natural Science Foundation of Hubei Province (Hubei Provincial Natural Science Foundation)
- 22238009/National Natural Science Foundation of China (National Science Foundation of China)
- 22278324/National Natural Science Foundation of China (National Science Foundation of China)
- 52073223/National Natural Science Foundation of China (National Science Foundation of China)
- 52322214/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

