IGFBP5 is released by senescent cells and is internalized by healthy cells, promoting their senescence through interaction with retinoic receptors
- PMID: 38351010
- PMCID: PMC10863175
- DOI: 10.1186/s12964-024-01469-1
IGFBP5 is released by senescent cells and is internalized by healthy cells, promoting their senescence through interaction with retinoic receptors
Abstract
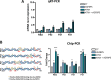
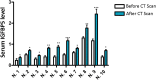
Cells that are exposed to harmful genetic damage, either from internal or external sources, may undergo senescence if they are unable to repair their DNA. Senescence, characterized by a state of irreversible growth arrest, can spread to neighboring cells through a process known as the senescence-associated secretory phenotype (SASP). This phenomenon contributes to both aging and the development of cancer. The SASP comprises a variety of factors that regulate numerous functions, including the induction of secondary senescence, modulation of immune system activity, remodeling of the extracellular matrix, alteration of tissue structure, and promotion of cancer progression. Identifying key factors within the SASP is crucial for understanding the underlying mechanisms of senescence and developing effective strategies to counteract cellular senescence. Our research has specifically focused on investigating the role of IGFBP5, a component of the SASP observed in various experimental models and conditions.Through our studies, we have demonstrated that IGFBP5 actively contributes to promoting senescence and can induce senescence in neighboring cells. We have gained valuable insights into the mechanisms through which IGFBP5 exerts its pro-senescence effects. These mechanisms include its release following genotoxic stress, involvement in signaling pathways mediated by reactive oxygen species and prostaglandins, internalization via specialized structures called caveolae, and interaction with a specific protein known as RARα. By uncovering these mechanisms, we have advanced our understanding of the intricate role of IGFBP5 in the senescence process. The significance of IGFBP5 as a pro-aging factor stems from an in vivo study we conducted on patients undergoing Computer Tomography analysis. In these patients, we observed an elevation in circulating IGFBP5 levels in response to radiation-induced organismal stress.Globally, our findings highlight the potential of IGFBP5 as a promising therapeutic target for age-related diseases and cancer.
Keywords: IGFBP; Mesenchymal stromal cells; SASP; Secretome; Senescence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
IGFBP7 is a key component of the senescence-associated secretory phenotype (SASP) that induces senescence in healthy cells by modulating the insulin, IGF, and activin A pathways.Cell Commun Signal. 2024 Nov 12;22(1):540. doi: 10.1186/s12964-024-01921-2. Cell Commun Signal. 2024. PMID: 39533382 Free PMC article.
-
Keeping the senescence secretome under control: Molecular reins on the senescence-associated secretory phenotype.Exp Gerontol. 2016 Sep;82:39-49. doi: 10.1016/j.exger.2016.05.010. Epub 2016 May 25. Exp Gerontol. 2016. PMID: 27235851 Review.
-
Increase of circulating IGFBP-4 following genotoxic stress and its implication for senescence.Elife. 2020 Mar 30;9:e54523. doi: 10.7554/eLife.54523. Elife. 2020. PMID: 32223893 Free PMC article.
-
Potential Regulators of the Senescence-Associated Secretory Phenotype During Senescence and Aging.J Gerontol A Biol Sci Med Sci. 2022 Nov 21;77(11):2207-2218. doi: 10.1093/gerona/glac097. J Gerontol A Biol Sci Med Sci. 2022. PMID: 35524726 Review.
-
Meta-analysis of senescent cell secretomes to identify common and specific features of the different senescent phenotypes: a tool for developing new senotherapeutics.Cell Commun Signal. 2023 Sep 28;21(1):262. doi: 10.1186/s12964-023-01280-4. Cell Commun Signal. 2023. PMID: 37770897 Free PMC article.
Cited by
-
When therapy-induced senescence meets tumors: A double-edged sword: A review.Medicine (Baltimore). 2025 Jun 13;104(24):e42886. doi: 10.1097/MD.0000000000042886. Medicine (Baltimore). 2025. PMID: 40527771 Free PMC article. Review.
-
Methods to Investigate the Secretome of Senescent Cells.Methods Protoc. 2024 Jul 2;7(4):52. doi: 10.3390/mps7040052. Methods Protoc. 2024. PMID: 39051266 Free PMC article. Review.
-
The Matrix Protein Tropoelastin Prolongs Mesenchymal Stromal Cell Vitality and Delays Senescence During Replicative Aging.Adv Sci (Weinh). 2024 Oct;11(39):e2402168. doi: 10.1002/advs.202402168. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39120048 Free PMC article.
-
Type 1 diabetes impairs the activity of rat testicular somatic and germ cells through NRF2/NLRP3 pathway-mediated oxidative stress.Front Endocrinol (Lausanne). 2024 May 16;15:1399256. doi: 10.3389/fendo.2024.1399256. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38818504 Free PMC article.
-
Deciphering the role of IGFBP5 in delaying fibrosis and sarcopenia in aging skeletal muscle: therapeutic implications and molecular mechanisms.Front Pharmacol. 2025 Mar 12;16:1557703. doi: 10.3389/fphar.2025.1557703. eCollection 2025. Front Pharmacol. 2025. PMID: 40144669 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

