ROS-mediated up-regulation of SAE1 by Helicobacter pylori promotes human gastric tumor genesis and progression
- PMID: 38351014
- PMCID: PMC10863176
- DOI: 10.1186/s12967-024-04913-5
ROS-mediated up-regulation of SAE1 by Helicobacter pylori promotes human gastric tumor genesis and progression
Abstract
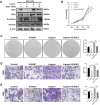
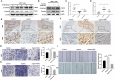
Helicobacter pylori (H. pylori) is a major risk factor of gastric cancer (GC). The SUMO-activating enzyme SAE1(SUMO-activating enzyme subunit 1), which is indispensable for protein SUMOylation, involves in human tumorigenesis. In this study, we used the TIMER and TCGA database to explore the SAE1 expression in GC and normal tissues and Kaplan-Meier Plotter platform for survival analysis of GC patients. GC tissue microarray and gastric samples from patients who underwent endoscopic treatment were employed to detect the SAE1expression. Our results showed that SAE1 was overexpressed in GC tissues and higher SAE1 expression was associated with worse clinical characteristics of GC patients. Cell and animal models showed that H. pylori infection upregulated SAE1, SUMO1, and SUMO2/3 protein expression. Functional assays suggested that suppression of SAE1 attenuated epithelial-mesenchymal transition (EMT) biomarkers and cell proliferation abilities induced by H. pylori. Cell and animal models of ROS inhibition in H. pylori showed that ROS could mediate the H. pylori-induced upregulation of SAE1, SUMO1, and SUMO2/3 protein. RNA sequencing was performed and suggested that knockdown of SAE1 could exert an impact on IGF-1 expression. General, increased SUMOylation modification is involved in H. pylori-induced GC.
Keywords: EMT; Gastric cancer; Helicobacter pylori; ROS; SAE1; SUMOylation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

