Binding kinetics drive G protein subtype selectivity at the β1-adrenergic receptor
- PMID: 38351103
- PMCID: PMC10864275
- DOI: 10.1038/s41467-024-45680-7
Binding kinetics drive G protein subtype selectivity at the β1-adrenergic receptor
Abstract
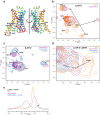
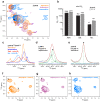
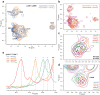
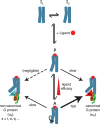
G protein-coupled receptors (GPCRs) bind to different G protein α-subtypes with varying degrees of selectivity. The mechanism by which GPCRs achieve this selectivity is still unclear. Using 13C methyl methionine and 19F NMR, we investigate the agonist-bound active state of β1AR and its ternary complexes with different G proteins in solution. We find the receptor in the ternary complexes adopts very similar conformations. In contrast, the full agonist-bound receptor active state assumes a conformation differing from previously characterised activation intermediates or from β1AR in ternary complexes. Assessing the kinetics of binding for the agonist-bound receptor with different G proteins, we find the increased affinity of β1AR for Gs results from its much faster association with the receptor. Consequently, we suggest a kinetic-driven selectivity gate between canonical and secondary coupling which arises from differential favourability of G protein binding to the agonist-bound receptor active state.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
An inactive receptor-G protein complex maintains the dynamic range of agonist-induced signaling.Proc Natl Acad Sci U S A. 2020 Dec 1;117(48):30755-30762. doi: 10.1073/pnas.2010801117. Epub 2020 Nov 16. Proc Natl Acad Sci U S A. 2020. PMID: 33199589 Free PMC article.
-
PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling.Nature. 2018 Jul;559(7714):423-427. doi: 10.1038/s41586-018-0325-6. Epub 2018 Jul 11. Nature. 2018. PMID: 29995853 Free PMC article.
-
Conformational plasticity of ligand-bound and ternary GPCR complexes studied by 19F NMR of the β1-adrenergic receptor.Nat Commun. 2020 Feb 3;11(1):669. doi: 10.1038/s41467-020-14526-3. Nat Commun. 2020. PMID: 32015348 Free PMC article.
-
G protein activation by G protein coupled receptors: ternary complex formation or catalyzed reaction?Biochem Pharmacol. 2004 Sep 1;68(5):799-806. doi: 10.1016/j.bcp.2004.05.044. Biochem Pharmacol. 2004. PMID: 15294442 Review.
-
Mechanistic insights into GPCR-G protein interactions.Curr Opin Struct Biol. 2016 Dec;41:247-254. doi: 10.1016/j.sbi.2016.11.005. Epub 2016 Nov 18. Curr Opin Struct Biol. 2016. PMID: 27871057 Free PMC article. Review.
Cited by
-
G Protein Selectivity in Dopamine Receptors is Determined before GDP Release.Biochemistry. 2025 Jun 3;64(11):2439-2454. doi: 10.1021/acs.biochem.4c00779. Epub 2025 May 13. Biochemistry. 2025. PMID: 40358213 Free PMC article.
-
NMR spectroscopy reveals insights into mechanisms of GPCR signaling.Trends Biochem Sci. 2025 Jan;50(1):84-85. doi: 10.1016/j.tibs.2024.10.007. Epub 2024 Nov 9. Trends Biochem Sci. 2025. PMID: 39523165 No abstract available.
-
Agonist efficacy at the β2AR is driven by the faster association rate of the Gs protein.Front Pharmacol. 2025 Mar 27;16:1367991. doi: 10.3389/fphar.2025.1367991. eCollection 2025. Front Pharmacol. 2025. PMID: 40213684 Free PMC article.
-
Functional dynamics of G protein-coupled receptors reveal new routes for drug discovery.Nat Rev Drug Discov. 2025 Apr;24(4):251-275. doi: 10.1038/s41573-024-01083-3. Epub 2025 Jan 2. Nat Rev Drug Discov. 2025. PMID: 39747671 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

