Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: a phase 2 umbrella trial
- PMID: 38351187
- PMCID: PMC10957481
- DOI: 10.1038/s41591-024-02808-y
Biomarker-directed targeted therapy plus durvalumab in advanced non-small-cell lung cancer: a phase 2 umbrella trial
Abstract
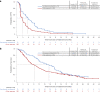
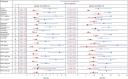
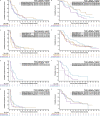
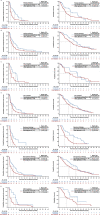
For patients with non-small-cell lung cancer (NSCLC) tumors without currently targetable molecular alterations, standard-of-care treatment is immunotherapy with anti-PD-(L)1 checkpoint inhibitors, alone or with platinum-doublet therapy. However, not all patients derive durable benefit and resistance to immune checkpoint blockade is common. Understanding mechanisms of resistance-which can include defects in DNA damage response and repair pathways, alterations or functional mutations in STK11/LKB1, alterations in antigen-presentation pathways, and immunosuppressive cellular subsets within the tumor microenvironment-and developing effective therapies to overcome them, remains an unmet need. Here the phase 2 umbrella HUDSON study evaluated rational combination regimens for advanced NSCLC following failure of anti-PD-(L)1-containing immunotherapy and platinum-doublet therapy. A total of 268 patients received durvalumab (anti-PD-L1 monoclonal antibody)-ceralasertib (ATR kinase inhibitor), durvalumab-olaparib (PARP inhibitor), durvalumab-danvatirsen (STAT3 antisense oligonucleotide) or durvalumab-oleclumab (anti-CD73 monoclonal antibody). Greatest clinical benefit was observed with durvalumab-ceralasertib; objective response rate (primary outcome) was 13.9% (11/79) versus 2.6% (5/189) with other regimens, pooled, median progression-free survival (secondary outcome) was 5.8 (80% confidence interval 4.6-7.4) versus 2.7 (1.8-2.8) months, and median overall survival (secondary outcome) was 17.4 (14.1-20.3) versus 9.4 (7.5-10.6) months. Benefit with durvalumab-ceralasertib was consistent across known immunotherapy-refractory subgroups. In ATM-altered patients hypothesized to harbor vulnerability to ATR inhibition, objective response rate was 26.1% (6/23) and median progression-free survival/median overall survival were 8.4/22.8 months. Durvalumab-ceralasertib safety/tolerability profile was manageable. Biomarker analyses suggested that anti-PD-L1/ATR inhibition induced immune changes that reinvigorated antitumor immunity. Durvalumab-ceralasertib is under further investigation in immunotherapy-refractory NSCLC.ClinicalTrials.gov identifier: NCT03334617.
© 2024. The Author(s).
Conflict of interest statement
B.B. has received grants or funds from Abbvie, Amgen, AstraZeneca, Chugai Pharmaceutical, Daiichi Sankyo, Ellipse Pharma, EISAI, Genmab, Genzyme Corporation, Hedera Dx, Inivata, IPSEN, Janssen, MSD, Pharmamar, Roche-Genentech, Sanofi, Socar Research, Tahio Oncology and Turning Point Therapeutics. E.P.-T. has participated in advisory councils or committees for AstraZeneca, Bristol Myers Squibb, Takeda and Sanofi. K.P. has participated in an advisory council or committee (steering committee) for and received grants or funds (out of this study) from AstraZeneca. S.H. has participated in advisory boards for MSD, Roche and AstraZeneca, and has received institutional grants or funds from AstraZeneca, GSK, Chiesi and Menarini Pharma. P.M.F. has received consulting fees from AstraZeneca, Bristol Myers Squibb, Novartis, Merck, Genentech, G1, F Star, Regeneron, Janssen, Sanofi, Amgen, Fosun, Teva, Synthekine, Flame, Iteos and Tavotek, and grants or funds from AstraZeneca, Bristol Myers Squibb, Regeneron, Novartis and BioNTech. M.J.H. has participated in advisory councils or committees for Bristol Myers Squibb, AstraZeneca, MSD, Lilly and Roche. M.M.A. has performed consulting or advisory roles with Merck, Pfizer, Bristol Myers Squibb, Foundation Medicine, Novartis, Gritstone Oncology, Mirati Therapeutics, EMD Serono, AstraZeneca, Instil Bio, Regeneron and Janssen, and has received institutional research funding from Genentech/Roche, Lilly, AstraZeneca, Bristol Myers Squibb, Amgen and Affini-T Therapeutics. M.T. has participated in advisory councils or committees for and received honoraria and consulting fees from AstraZeneca, Roche, Bristol Myers Squibb, MSD, Novartis, Sanofi, Takeda, Pfizer, Merck, Daiichi Sankyo, Janssen, Lilly and Boehringer Ingelheim, and has received grants or funds from AstraZeneca, Roche and Takeda. G.G. has received honoraria from AstraZeneca. P.W.-P. has participated in advisory councils or committees for AstraZeneca, Bristol Myers Squibb, Sanofi, Roche, Novartis, Jazz Pharmaceuticals and Pfizer, and has received honoraria from Bayer, Sanofi and Merck. F.A.S. has participated in advisory boards for and received honoraria and consulting fees from AstraZeneca, and their institution has received clinical trial payments from AstraZeneca. M.F. has participated in advisory councils or committees for AstraZeneca, Bristol Myers Squibb and Takeda, has received honoraria from AstraZeneca and Bristol Myers Squibb, and has received grants or funds from AstraZeneca. P.C. has participated in advisory councils or committees for AstraZeneca and Novartis, received honoraria from AstraZeneca, Janssen, Eli Lilly, GSK, Merck, Sanofi and Amgen, and received consulting fees from AstraZeneca, Bristol Myers Squibb, Roche, Amgen, Pfizer, Novartis and Merck. Q.S.C.C. has received honoraria and consulting fees from Abbvie, Amgen, AnHeart, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Esperas Pharma, Eisai, Jazz Pharmaceutical, Janssen/Johnson and Johnson, Merck, Novartis, Ocellaris, Pfizer, Roche and Takeda. S.-W.K. has no conflicts of interest to declare. D.M. has received consulting fees from Abbvie, Arcus, Mirati and Lilly, and grants or funds from Merck, AstraZeneca, Incyte, Bristol Myers Squibb, Surface, Epicentrx, Boehringer Ingelheim, Y-mabs, Roche, Lilly, Pfizer and Novartis. M.L.J. has received institutional research funding from AbbVie, Acerta, Adaptimmune, Amgen, Apexigen, Arcus Biosciences, Array BioPharma, Artios Pharma, AstraZeneca, Atreca, BeiGene, BerGenBio, BioAtla, Black Diamond, Boehringer Ingelheim, Bristol Myers Squibb, Calithera Biosciences, Carisma Therapeutics, Checkpoint Therapeutics, City of Hope National Medical Center, Corvus Pharmaceuticals, Curis, CytomX, Daiichi Sankyo, Dracen Pharmaceuticals, Dynavax, Lilly, Elicio Therapeutics, EMD Serono, EQRx, Erasca, Exelixis, Fate Therapeutics, Genentech/Roche, Genmab, Genocea Biosciences, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Harpoon, Helsinn Healthcare SA, Hengrui Therapeutics, Hutchison MediPharma, IDEAYA Biosciences, IGM Biosciences, Immunitas Therapeutics, Immunocore, Incyte, Janssen, Jounce Therapeutics, Kadmon Pharmaceuticals, Kartos Therapeutics, Loxo Oncology, Lycera, Memorial Sloan-Kettering, Merck, Merus, Mirati Therapeutics, Mythic Therapeutics, NeoImmune Tech, Neovia Oncology, Novartis, Numab Therapeutics, Nuvalent, OncoMed Pharmaceuticals, Palleon Pharmaceuticals, Pfizer, PMV Pharmaceuticals, Rain Therapeutics, RasCal Therapeutics, Regeneron Pharmaceuticals, Relay Therapeutics, Revolution Medicines, Ribon Therapeutics, Rubius Therapeutics, Sanofi, Seven and Eight Biopharmaceuticals/Birdie Biopharmaceuticals, Shattuck Labs, Silicon Therapeutics, Stem CentRx, Syndax Pharmaceuticals, Takeda Pharmaceuticals, Tarveda, TCR2 Therapeutics, Tempest Therapeutics, Tizona Therapeutics, TMUNITY Therapeutics, Turning Point Therapeutics, University of Michigan, Vyriad, WindMIL Therapeutics and Y-mAbs Therapeutics; and has received institutional payments for consulting or advisory roles for AbbVie, Amgen, Arcus Biosciences, Arrivent, Astellas, AstraZeneca, Black Diamond, Boehringer Ingelheim, Calithera Biosciences, Daiichi Sankyo, EcoR1, Genentech/Roche, Genmab, Genocea Biosciences, Gilead Sciences, GlaxoSmithKline, Gritstone Oncology, Ideaya Biosciences, Immunocore, iTeos, Janssen, Jazz Pharmaceuticals, Merck, Mirati Therapeutics, Molecular Axiom, Normunity, Novartis, Oncorus, Pyramid Biosciences, Regeneron Pharmaceuticals, Revolution Medicines, Sanofi-Aventis, SeaGen, Synthekine, Takeda Pharmaceuticals, Turning Point Therapeutics and VBL Therapeutics. S.C. has participated in advisory councils or committees for AstraZeneca, Bristol Myers Squibb, MSD and Sanofi. D.-W.K. has received research funding to their institution from Alpha Biopharma, Amgen, AstraZeneca/Medimmune, Boehringer Ingelheim, Bridge BioTherapeutics, Chong Keun Dang, Daiichi Sankyo, GSK, Hanmi, InnoN, Janssen, Merck, Merus, Mirati Therapeutics, MSD, Novartis, ONO Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery and Yuhan, and has received medical writing assistance from Amgen, AstraZeneca, Boehringer Ingelheim, Bridge BioTherapeutics, Chong Keun Dang, Daiichi Sankyo, GSK, Janssen, Merus, Mirati Therapeutics, MSD, Meck, Novartis, Pfizer, Roche, Takeda and Yuhan. M.T.M. has participated in advisory councils or committees for MSD, Takeda, Bayer, Amgen and Novartis, has received honoraria from Bristol Myers Squibb, MSD, Takeda, AstraZeneca, Roche and AbbVie, has received consulting fees from MSD and AstraZeneca, and has received grants or funds from AstraZeneca. D.V. has received honoraria from Bristol Myers Squibb, AstraZeneca, Pfizer, Novartis, Roche and Daichii. B.A., R.H., H.J.A., S.K., A.R., D.L.R., M.R.K., J.P.C., J.C.B., E.D., R.K., M.D., P.J.J., S.I., S.T.B. and J.C. are employees of and may own stock/shares in AstraZeneca. D.L.R. owns stocks or shares in Myriad Genetics. J.V.H. has participated in advisory councils or committees for AstraZeneca, Bristol Myers Squibb, Genentech/Roche, EMD Serono, GlaxoSmithKline, Hengrui Therapeutics, Eli Lilly, Novartis, Spectrum, Sanofi, Takeda, Mirati Therapeutics, Janssen Global Services and Leads Biolabs, has received honoraria from AstraZeneca, Bristol Myers Squibb, Genentech/Roche, EMD Serono, GlaxoSmithKline, Hengrui Therapeutics, Eli Lilly, Novartis, Spectrum, Sanofi, Takeda, Mirati Therapeutics, Janssen Global Services, Leads Biolabs and Boehringer Ingelheim, has received grants or funds from AstraZeneca, Spectrum, Boehringer Ingelheim and Takeda, and has received licensing fees from Spectrum.
Figures






Comment in
-
Novel immune checkpoint inhibitor strategies in advanced non-small cell lung cancer: towards biomarker-driven therapies?Transl Lung Cancer Res. 2025 Feb 28;14(2):328-333. doi: 10.21037/tlcr-24-966. Epub 2025 Feb 24. Transl Lung Cancer Res. 2025. PMID: 40114950 Free PMC article. No abstract available.
References
-
- NCCN clinical practice guidelines in oncology: non-small cell lung cancer, version 3.2022. National Comprehensive Cancer Networkhttps://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (2022). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

