Transforming growth factor-β receptors: versatile mechanisms of ligand activation
- PMID: 38351317
- PMCID: PMC11192764
- DOI: 10.1038/s41401-024-01235-6
Transforming growth factor-β receptors: versatile mechanisms of ligand activation
Abstract
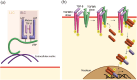
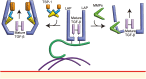
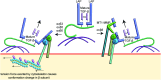
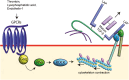
Transforming growth factor-β (TGF-β) signaling is initiated by activation of transmembrane TGF-β receptors (TGFBR), which deploys Smad2/3 transcription factors to control cellular responses. Failure or dysregulation in the TGF-β signaling pathways leads to pathological conditions. TGF-β signaling is regulated at different levels along the pathways and begins with the liberation of TGF-β ligand from its latent form. The mechanisms of TGFBR activation display selectivity to cell types, agonists, and TGF-β isoforms, enabling precise control of TGF-β signals. In addition, the cell surface compartments used to release active TGF-β are surprisingly vibrant, using thrombospondins, integrins, matrix metalloproteinases and reactive oxygen species. The scope of TGFBR activation is further unfolded with the discovery of TGFBR activation initiated by other signaling pathways. The unique combination of mechanisms works in series to trigger TGFBR activation, which can be explored as therapeutic targets. This comprehensive review provides valuable insights into the diverse mechanisms underpinning TGFBR activation, shedding light on potential avenues for therapeutic exploration.
Keywords: TGFBR; integrins; matrix metalloproteinases; receptor Smads; thrombospondins; transactivation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Transforming growth factor-β (TGF-β)-induced up-regulation of TGF-β receptors at the cell surface amplifies the TGF-β response.J Biol Chem. 2019 May 24;294(21):8490-8504. doi: 10.1074/jbc.RA118.005763. Epub 2019 Apr 4. J Biol Chem. 2019. PMID: 30948511 Free PMC article.
-
TGF-β receptors: In and beyond TGF-β signaling.Cell Signal. 2018 Dec;52:112-120. doi: 10.1016/j.cellsig.2018.09.002. Epub 2018 Sep 7. Cell Signal. 2018. PMID: 30184463 Review.
-
NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor.Biochem J. 2005 Mar 15;386(Pt 3):461-70. doi: 10.1042/BJ20040738. Biochem J. 2005. PMID: 15496141 Free PMC article.
-
Feedback regulation of TGF-β signaling.Acta Biochim Biophys Sin (Shanghai). 2018 Jan 1;50(1):37-50. doi: 10.1093/abbs/gmx129. Acta Biochim Biophys Sin (Shanghai). 2018. PMID: 29228156 Review.
-
The transforming growth factor-beta/Smad2,3 signalling axis is impaired in experimental pulmonary hypertension.Eur Respir J. 2007 Jun;29(6):1094-104. doi: 10.1183/09031936.00138206. Epub 2007 Mar 28. Eur Respir J. 2007. PMID: 17392319
Cited by
-
Cardiac Fibrosis in the Multi-Omics Era: Implications for Heart Failure.Circ Res. 2025 Mar 28;136(7):773-802. doi: 10.1161/CIRCRESAHA.124.325402. Epub 2025 Mar 27. Circ Res. 2025. PMID: 40146800 Free PMC article. Review.
-
Transcriptome Analysis of TGFBI Knockdown vs Normal Corneal Epithelial Cells: Implications for TGFBI Corneal Dystrophy Treatment.Biochem Genet. 2025 Jul 15. doi: 10.1007/s10528-025-11191-3. Online ahead of print. Biochem Genet. 2025. PMID: 40665122
-
Neu1 deficiency and fibrotic lymph node microenvironment lead to imbalance in M1/M2 macrophage polarization.Front Immunol. 2024 Sep 13;15:1462853. doi: 10.3389/fimmu.2024.1462853. eCollection 2024. Front Immunol. 2024. PMID: 39346907 Free PMC article.
-
Tumor microenvironment and immune-related myositis: addressing muscle wasting in cancer immunotherapy.Front Immunol. 2025 May 2;16:1580108. doi: 10.3389/fimmu.2025.1580108. eCollection 2025. Front Immunol. 2025. PMID: 40386783 Free PMC article. Review.
-
Progress of microneedle targeted modulation technology in the reconstruction of immune microenvironment in diabetic wounds.Eur J Med Res. 2025 May 20;30(1):405. doi: 10.1186/s40001-025-02667-4. Eur J Med Res. 2025. PMID: 40394697 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

