The development of early human lymphatic vessels as characterized by lymphatic endothelial markers
- PMID: 38351385
- PMCID: PMC10907744
- DOI: 10.1038/s44318-024-00045-0
The development of early human lymphatic vessels as characterized by lymphatic endothelial markers
Abstract
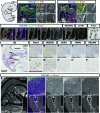
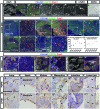
Lymphatic vessel development studies in mice and zebrafish models have demonstrated that lymphatic endothelial cells (LECs) predominantly differentiate from venous endothelial cells via the expression of the transcription factor Prox1. However, LECs can also be generated from undifferentiated mesoderm, suggesting potential diversity in their precursor cell origins depending on the organ or anatomical location. Despite these advances, recapitulating human lymphatic malformations in animal models has been difficult, and considering lymphatic vasculature function varies widely between species, analysis of development directly in humans is needed. Here, we examined early lymphatic development in humans by analyzing the histology of 31 embryos and three 9-week-old fetuses. We found that human embryonic cardinal veins, which converged to form initial lymph sacs, produce Prox1-expressing LECs. Furthermore, we describe the lymphatic vessel development in various organs and observe organ-specific differences. These characterizations of the early development of human lymphatic vessels should help to better understand the evolution and phylogenetic relationships of lymphatic systems, and their roles in human disease.
Keywords: Cellular Origin of Lymphatic Endothelial Cells; Human Embryos; Lymphatic Vessel Development.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

