Predicting residual cholesteatoma with the Potsic staging system still lacks evidence: a systematic review and meta-analysis
- PMID: 38351408
- PMCID: PMC11211107
- DOI: 10.1007/s00405-024-08478-3
Predicting residual cholesteatoma with the Potsic staging system still lacks evidence: a systematic review and meta-analysis
Abstract
Purpose: To investigate the rate of residual disease in the Potsic staging system for congenital cholesteatomas.
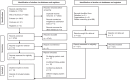
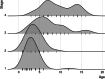
Methods: A protocol registration was published on PROSPERO (CRD42022383932), describing residual disease as a primary outcome and hearing improvement as secondary. A systematic search was performed in four databases (PubMed, Embase, Cochrane Library, Web of Science) on December 14, 2022. Articles were included if cholesteatomas were staged according to the Potsic system and follow-up duration was documented. Risk of bias was evaluated using the Quality In Prognosis Studies (QUIPS) tool. In the statistical synthesis a random effects model was used. Between-study heterogeneity was assessed using I2.
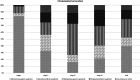
Results: Thirteen articles were found to be eligible for systematic review and seven were included in the meta-analysis section. All records were retrospective cohort studies with high risk of bias. Regarding the proportions of residual disease, analysis using the χ2 test showed no statistically significant difference between Potsic stages after a follow-up of minimum one year (stage I 0.06 (confidence interval (CI) 0.01-0.33); stage II 0.20 (CI 0.09-0.38); stage III 0.06 (CI 0.00-0.61); stage IV: 0.17 (CI 0.01-0.81)). Postoperative and preoperative hearing outcomes could not be analyzed due to varied reporting. Results on cholesteatoma location and mean age at staging were consistent with those previously published.
Conclusion: No statistically significant difference was found in the proportions of residual disease between Potsic stages, thus the staging system's applicability for outcome prediction could not be proven based on the available data. Targeted studies are needed for a higher level of evidence.
Keywords: Congenital cholesteatoma; Potsic; Residual cholesteatoma.
© 2024. The Author(s).
Conflict of interest statement
The authors have no competing non- or financial interests to declare.
Figures

References
-
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

