The role of programmed death receptor (PD-)1/PD-ligand (L)1 in periodontitis and cancer
- PMID: 38351432
- PMCID: PMC11579837
- DOI: 10.1111/prd.12548
The role of programmed death receptor (PD-)1/PD-ligand (L)1 in periodontitis and cancer
Abstract
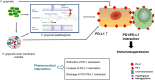
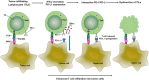
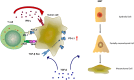
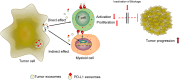
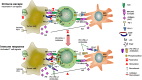
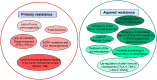
The programmed-death-ligand-1 (PD-L1) is an immune-modulating molecule that is constitutively expressed on various immune cells, different epithelial cells and a multitude of cancer cells. It is a costimulatory molecule that may impair T-cell mediated immune response. Ligation to the programmed-death-receptor (PD)-1, on activated T-cells and further triggering of the related signaling pathways can induce T-cells apoptosis or anergy. The upregulation of PD-L1 in various cancer types, including oral squamous cell carcinomas, was demonstrated and has been linked to immune escape of tumors and poor prognosis. A bidirectional relationship exists between the increased PD-L1 expression and periodontitis as well as the epithelial-mesenchymal transition (EMT), a process of interconversion of epithelial cells to mesenchymal cells that may induce immune escape of tumors. Interaction between exosomal PD-L1 and PD-1 on T-cells may cause immunosuppression by blocking the activation and proliferation of T-cells. The efficacy and importance of treatment with PD-1/PD-L1 checkpoint inhibitors and their prognostic influence on human cancers was demonstrated. Regarding PD-1/PD-L1 checkpoint inhibitors, resistances exist or may develop, basing on various factors. Further investigations of the underlying mechanisms will help to overcome the therapeutic limitations that result from resistances and to develop new strategies for the treatment of cancer.
Keywords: EMT; PD‐L1; cancer; exosomes; immune checkpoint; immune escape; periodontitis.
© 2024 The Authors. Periodontology 2000 published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Programmed death receptor (PD-)1/PD-ligand (L)1 in urological cancers : the "all-around warrior" in immunotherapy.Mol Cancer. 2024 Sep 2;23(1):183. doi: 10.1186/s12943-024-02095-8. Mol Cancer. 2024. PMID: 39223527 Free PMC article. Review.
-
Periodontitis promotes tumor growth and immune evasion via PD-1/PD-L1.Cancer Immunol Immunother. 2024 Nov 13;74(1):22. doi: 10.1007/s00262-024-03865-5. Cancer Immunol Immunother. 2024. PMID: 39535607 Free PMC article.
-
Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion.Cancer Lett. 2020 Jan 1;468:72-81. doi: 10.1016/j.canlet.2019.10.013. Epub 2019 Oct 9. Cancer Lett. 2020. PMID: 31605776 Review.
-
Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: a key role of exosomal PD-L1.J Immunother Cancer. 2021 Jan;9(1):e001698. doi: 10.1136/jitc-2020-001698. J Immunother Cancer. 2021. PMID: 33472857 Free PMC article. Review.
-
PD-L1-Mediated Immunosuppression in Oral Squamous Cell Carcinoma: Relationship With Macrophage Infiltration and Epithelial to Mesenchymal Transition Markers.Front Immunol. 2021 Sep 6;12:693881. doi: 10.3389/fimmu.2021.693881. eCollection 2021. Front Immunol. 2021. PMID: 34552581 Free PMC article.
Cited by
-
Causal associations of MICB, CTSA, and MMP9 proteins with oral cancer: Mendelian randomization study.Sci Rep. 2024 Oct 27;14(1):25645. doi: 10.1038/s41598-024-77042-0. Sci Rep. 2024. PMID: 39465349 Free PMC article.
-
Immunocytes interact directly with cancer cells in the tumor microenvironment: one coin with two sides and future perspectives.Front Immunol. 2024 May 22;15:1388176. doi: 10.3389/fimmu.2024.1388176. eCollection 2024. Front Immunol. 2024. PMID: 38840908 Free PMC article. Review.
-
Porphyromonas gingivalis induces Zbp1-mediated macrophages PANoptosis in periodonitis pathophysiology.Exp Mol Med. 2025 May;57(5):964-978. doi: 10.1038/s12276-025-01443-y. Epub 2025 May 1. Exp Mol Med. 2025. PMID: 40307566 Free PMC article.
References
-
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481‐490. - PubMed
-
- Larsen T, Fiehn NE. Dental biofilm infections—an update. APMIS. 2017;125:376‐384. - PubMed
-
- Picolos DK, Lerche‐Sehm J, Abron A, Fine JB, Papapanou PN. Infection patterns in chronic and aggressive periodontitis. J Clin Periodontol. 2005;32:1055‐1061. - PubMed
-
- Dong H, Strome SE, Salomao DR, et al. Tumor‐associated B7‐H1 promotes T‐cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793‐800. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

