Evaluating Chlamydia trachomatis and Neisseria gonorrhoeae screening and treatment among asymptomatic pregnant women to prevent preterm birth and low birthweight in Gaborone, Botswana: A secondary analysis from a non-randomised, cluster-controlled trial
- PMID: 38351649
- PMCID: PMC11500666
- DOI: 10.1111/1471-0528.17775
Evaluating Chlamydia trachomatis and Neisseria gonorrhoeae screening and treatment among asymptomatic pregnant women to prevent preterm birth and low birthweight in Gaborone, Botswana: A secondary analysis from a non-randomised, cluster-controlled trial
Abstract
Objective: To evaluate the impact of screening and treating asymptomatic pregnant women for Chlamydia (C.) trachomatis and Neisseria (N.) gonorrhoeae infections on the frequency of preterm birth or low birthweight infants in Botswana.
Design: Non-randomised, cluster-controlled trial.
Setting: Four antenatal care clinics in Gaborone, Botswana.
Population: Pregnant women aged ≥15 years, attending a first antenatal care visit, ≤27 weeks of gestation and without urogenital symptoms were eligible.
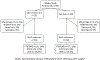
Methods: Participants in the intervention clinics received screening (GeneXpert®, Cepheid) during pregnancy and at the postnatal visit. Participants in the standard-of-care clinics received screening at the postnatal visit only. We used multivariable logistic regression and post-estimation predictive margins analysis. Post-hoc analysis was conducted among sub-samples stratified by parity.
Main outcome measures: Preterm birth (<37 weeks of gestation) and low birthweight (<2500 g).
Results: After controlling for parity, hypertension, antenatal care visits and clinic site, the predicted prevalence of preterm birth or low birthweight was lower in the intervention arm (11%) compared with the standard-of-care arm (16%) (adjusted odds ratio [aOR] 0.59; 95% confidence interval [CI] 0.28-1.24). In post-hoc analysis, the intervention was more effective than the standard-of-care (aOR 0.20; 95% CI 0.07-0.64) among nulliparous participants.
Conclusion: A C. trachomatis and N. gonorrhoeae infection screening and treatment intervention among asymptomatic pregnant women may have reduced preterm birth or low birthweight outcomes, but results were not statistically significant. Post-hoc analysis found that the intervention reduced adverse outcomes among nulliparous participants.
Keywords: Chlamydia trachomatis; Neisseria gonorrhoeae; low birthweight; pregnancy; preterm birth; sexually transmitted infections; syndromic management.
© 2024 The Authors. BJOG: An International Journal of Obstetrics and Gynaecology published by John Wiley & Sons Ltd.
Conflict of interest statement
Disclosure of interests
Dr Klausner reports personal fees from Cepheid, during the conduct of the study, and personal fees from Danaher, outside the submitted work. None of the other authors declares a conflict of interest. Completed disclosure of interests forms are available to view online as supporting information.
Figures
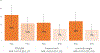
Similar articles
-
Effect of antenatal Chlamydia trachomatis and Neisseria gonorrhoeae screening on postdelivery prevalence and vertical transmission in Gaborone, Botswana: findings from an exploratory study.Sex Transm Infect. 2025 Mar 24;101(2):81-87. doi: 10.1136/sextrans-2023-055965. Sex Transm Infect. 2025. PMID: 39366745 Clinical Trial.
-
Point-of-care testing and treatment of sexually transmitted and genital infections to improve birth outcomes in high-burden, low-resource settings (WANTAIM): a pragmatic cluster randomised crossover trial in Papua New Guinea.Lancet Glob Health. 2024 Apr;12(4):e641-e651. doi: 10.1016/S2214-109X(24)00004-4. Lancet Glob Health. 2024. PMID: 38485431 Clinical Trial.
-
Use of Expedited Partner Therapy for Pregnant Women Treated for Sexually Transmitted Infections in Gaborone, Botswana.Sex Transm Dis. 2024 May 1;51(5):331-336. doi: 10.1097/OLQ.0000000000001928. Epub 2024 Jan 23. Sex Transm Dis. 2024. PMID: 38301627 Clinical Trial.
-
Interventions for treating genital Chlamydia trachomatis infection in pregnancy.Cochrane Database Syst Rev. 2017 Sep 22;9(9):CD010485. doi: 10.1002/14651858.CD010485.pub2. Cochrane Database Syst Rev. 2017. PMID: 28937705 Free PMC article.
-
Strategies of testing for syphilis during pregnancy.Cochrane Database Syst Rev. 2014 Oct 29;2014(10):CD010385. doi: 10.1002/14651858.CD010385.pub2. Cochrane Database Syst Rev. 2014. PMID: 25352226 Free PMC article.
Cited by
-
The effect of STI screening during pregnancy on vertical transmission of HIV and adverse pregnancy outcomes in South Africa: a modelling study.J Int AIDS Soc. 2025 Feb;28(2):e26410. doi: 10.1002/jia2.26410. J Int AIDS Soc. 2025. PMID: 39865475 Free PMC article.
-
Effect of antenatal Chlamydia trachomatis and Neisseria gonorrhoeae screening on postdelivery prevalence and vertical transmission in Gaborone, Botswana: findings from an exploratory study.Sex Transm Infect. 2025 Mar 24;101(2):81-87. doi: 10.1136/sextrans-2023-055965. Sex Transm Infect. 2025. PMID: 39366745 Clinical Trial.
-
Intrapartum and postpartum antibiotic use in seven low- and middle-income countries: Findings from the A-PLUS trial.BJOG. 2025 Jan;132(1):72-80. doi: 10.1111/1471-0528.17930. Epub 2024 Aug 14. BJOG. 2025. PMID: 39140197 Clinical Trial.
-
Antenatal Screening for Sexually Transmitted Infections to Improve Maternal and Newborn Outcomes: An Update From 11 Low- and Middle-Income Countries.Sex Transm Dis. 2025 Mar 1;52(3):141-145. doi: 10.1097/OLQ.0000000000002100. Epub 2024 Nov 25. Sex Transm Dis. 2025. PMID: 39874241 Free PMC article.
References
-
- Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. The Lancet Child & adolescent health. 2022;6(2):106–15. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

