Clinical impact of pleural fluid carcinoembryonic antigen on therapeutic strategy and efficacy in lung adenocarcinoma patients with malignant pleural effusion
- PMID: 38351680
- PMCID: PMC10918375
- DOI: 10.3904/kjim.2023.309
Clinical impact of pleural fluid carcinoembryonic antigen on therapeutic strategy and efficacy in lung adenocarcinoma patients with malignant pleural effusion
Abstract
Background/aims: Epidermal growth factor receptor (EGFR) mutation is important in determining the treatment strategy for advanced lung cancer patients with malignant pleural effusion (MPE). Contrary to serum carcinoembryonic antigen (S-CEA) levels, the associations between pleural fluid CEA (PF-CEA) levels and EGFR mutation status as well as between PF-CEA levels and treatment efficacy have rarely been investigated in lung adenocarcinoma patients with MPE.
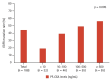
Methods: This retrospective study enrolled lung adenocarcinoma patients with MPE and available PF-CEA levels and EGFR mutation results. The patients were categorized based on PF-CEA levels: < 10 ng/mL, 10-100 ng/mL, 100-500 ng/mL, and ≥ 500 ng/mL. The association between PF-CEA levels and EGFR mutation status as well as their therapeutic impact on overall survival was compared among the four groups.
Results: This study included 188 patients. PF-CEA level was found to be an independent predictor of EGFR mutation but not S-CEA level. The EGFR mutation rates were higher as the PF-CEA levels increased, regardless of cytology results or sample types. Among EGFR-mutant lung adenocarcinoma patients receiving EGFR-tyrosine kinase inhibitor (TKI) treatment, those with high PF-CEA levels had significantly better survival outcomes than those with low PF-CEA levels.
Conclusion: High PF-CEA levels were associated with high EGFR mutation rate and may lead to a favorable clinical outcome of EGFR-TKI treatment in EGFR-mutant lung adenocarcinoma patients with MPE. These findings highlight the importance of actively investigating EGFR mutation detection in patients with suspected MPE and elevated PF-CEA levels despite negative cytology results.
Keywords: Epidermal growth factor receptor; Malignant pleural effusion; Mutation; Pleural fluid–carcinoembryonic antigen; Tyrosine kinase inhibitor.
Conflict of interest statement
The authors disclose no conflicts.
Figures


References
-
- Asciak R, Rahman NM. Malignant pleural effusion: from diagnostics to therapeutics. Clin Chest Med. 2018;39:181–193. - PubMed
-
- Wang S, Chen H, Zhong J, et al. Comparative study of EGFR mutations detected in malignant pleural effusion, plasma and tumor tissue in patients with adenocarcinoma of the lung. Lung Cancer. 2019;135:116–122. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
