Candidate Biomarkers for Targeting in Type 1 Diabetes; A Bioinformatic Analysis of Pancreatic Cell Surface Antigens
- PMID: 38351729
- PMCID: PMC10864774
- DOI: 10.22074/cellj.2023.1996297.1262
Candidate Biomarkers for Targeting in Type 1 Diabetes; A Bioinformatic Analysis of Pancreatic Cell Surface Antigens
Abstract
Objective: Type 1 diabetes (T1Ds) is an autoimmune disease in which the immune system invades and destroys insulin-producing cells. Nevertheless, at the time of diagnosis, about 30-40% of pancreatic beta cells are healthy and capable of producing insulin. Bi-specific antibodies, chimeric antigen receptor regulatory T cells (CAR-Treg cells), and labeled antibodies could be a new emerging option for the treatment or diagnosis of type I diabetic patients. The aim of the study is to choose appropriate cell surface antigens in the pancreas tissue for generating an antibody for type I diabetic patients.
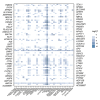
Materials and methods: In this bioinformatics study, we extracted pancreas-specific proteins from two large databases; the Human Protein Atlas (HPA) and Genotype-Tissue Expression (GTEx) Portal. Pancreatic-enriched genes were chosen and narrowed down by Protter software for the investigation of accessible extracellular domains. The immunohistochemistry (IHC) data of the protein atlas database were used to evaluate the protein expression of selected antigens. We explored the function of candidate antigens by using the GeneCards database to evaluate the potential dysfunction or activation/hyperactivation of antigens after antibody binding.
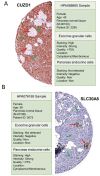
Results: The results showed 429 genes are highly expressed in the pancreas tissue. Also, eighteen genes encoded plasma membrane proteins that have high expression in the microarray (GEO) dataset. Our results introduced four structural proteins, including NPHS1, KIRREL2, GP2, and CUZD1, among all seventeen candidate proteins.
Conclusion: The presented antigens can potentially be used to produce specific pancreatic antibodies that guide CARTreg, bi-specific, or labeling molecules to the pancreas for treatment, detection, or other molecular targeted therapy scopes for type I diabetes.
Keywords: Bioinformatics; Cell Surface Antigens; Molecular Targeted Therapies; Pancreatic Islets; Type 1 Diabetes.
Figures



Similar articles
-
Rediscovery of the Anti-Pancreatic Antibodies and Evaluation of their Prognostic Value in a Prospective Clinical Cohort of Crohn's Patients: The Importance of Specific Target Antigens [GP2 and CUZD1].J Crohns Colitis. 2015 Aug;9(8):659-68. doi: 10.1093/ecco-jcc/jjv087. Epub 2015 May 12. J Crohns Colitis. 2015. PMID: 25968583 Clinical Trial.
-
Altered cellular localisation and expression, together with unconventional protein trafficking, of prion protein, PrPC, in type 1 diabetes.Diabetologia. 2021 Oct;64(10):2279-2291. doi: 10.1007/s00125-021-05501-8. Epub 2021 Jul 17. Diabetologia. 2021. PMID: 34274990 Free PMC article.
-
Kirrel2, a novel immunoglobulin superfamily gene expressed primarily in beta cells of the pancreatic islets.Genomics. 2003 Aug;82(2):130-42. doi: 10.1016/s0888-7543(03)00110-1. Genomics. 2003. PMID: 12837264
-
Gangliosides and autoimmune diabetes.Diabetes Metab Rev. 1997 Sep;13(3):163-79. doi: 10.1002/(sici)1099-0895(199709)13:3<163::aid-dmr189>3.0.co;2-z. Diabetes Metab Rev. 1997. PMID: 9307889 Review.
-
A comprehensive guide to antibody and T-cell responses in type 1 diabetes.Tissue Antigens. 2003 Nov;62(5):359-77. doi: 10.1034/j.1399-0039.2003.00152.x. Tissue Antigens. 2003. PMID: 14617043 Review.
Cited by
-
Redosing with Intralymphatic GAD-Alum in the Treatment of Type 1 Diabetes: The DIAGNODE-B Pilot Trial.Int J Mol Sci. 2025 Jan 4;26(1):374. doi: 10.3390/ijms26010374. Int J Mol Sci. 2025. PMID: 39796229 Free PMC article. Clinical Trial.
References
-
- Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol. 2019;15(11):635–650. - PubMed
-
- Jwad SM, AL-Fatlawi HY. Types of diabetes and their effect on the immune system. J Adv Pharm Pract. 2022;4(1):21–30.
-
- Labrijn AF, Janmaat ML, Reichert JM, Parren PWHI. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019;18(8):585–608. - PubMed
LinkOut - more resources
Full Text Sources
