Photochromic derivatives of indigo: historical overview of development, challenges and applications
- PMID: 38352070
- PMCID: PMC10862137
- DOI: 10.3762/bjoc.20.23
Photochromic derivatives of indigo: historical overview of development, challenges and applications
Abstract
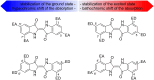
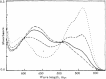
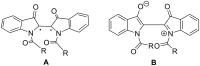
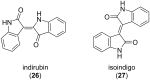
The importance of indigo dyes is constantly increasing with the evolution of novel textile materials and photochromic material technologies. The aim of this review article is to provide a comprehensive overview of the development of photochromic indigo derivatives from the first report on the photochromic N,N'-diacetylindigo in 1954 until now. We begin with the list of historical milestones in the development of photochromic indigo derivatives. Further, we provide a brief description of the synthetic procedures utilised to obtain indigo and its derivatives, outline the structural peculiarities, photophysical and photochemical properties of indigo and proceed with the detailed discussion of the photochromic indigo derivatives. Finally, we highlight the photochromism of the structural isomers of indigo (isoindigo and indirubin) and provide an overview of prospective applications of indigo photoswitches.
Keywords: E–Z isomerization; indigoid dyes; photochemistry; photophysics; photoswitching.
Copyright © 2024, Kaplan et al.
Figures












References
Publication types
LinkOut - more resources
Full Text Sources
