Toxoplasma-host endoplasmic reticulum interaction: How T. gondii activates unfolded protein response and modulates immune response
- PMID: 38352129
- PMCID: PMC10861954
- DOI: 10.1016/j.crmicr.2024.100223
Toxoplasma-host endoplasmic reticulum interaction: How T. gondii activates unfolded protein response and modulates immune response
Abstract
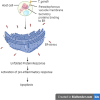
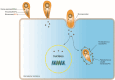
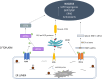
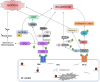
Toxoplasma gondii is a neurotropic single-celled zoonotic parasite that can infect human beings and animals. Infection with T. gondii is usually asymptomatic in immune-competent individual, however, it can cause symptomatic and life-threatening conditions in immunocompromised individuals and in developing foetuses. Although the mechanisms that allow T. gondii to persist in host cells are poorly understood, studies in animal models have greatly improved our understanding of Toxoplasma-host cell interaction and how this interaction modulates parasite proliferation and development, host immune response and virulence of the parasite. T. gondii is capable of recruiting the host endoplasmic reticulum (ER), suggesting it may influence the host ER function. Herein, we provide an overview of T. gondii infection and the role of host ER during stressed conditions. Furthermore, we highlight studies that explore T. gondii's interaction with the host ER. We delve into how this interaction activates the unfolded protein response (UPR) and ER stress-mediated apoptosis. Additionally, we examine how T. gondii exploits these pathways to its advantage.
Keywords: Apoptosis; ER–stress; Endoplasmic reticulum; Immune response; Toxoplasma gondii; UPR.
© 2024 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Amen O.M., Sarker S.D., Ghildyal R., Arya A. Endoplasmic reticulum stress activates unfolded protein response signaling and mediates inflammation, obesity, and cardiac dysfunction: therapeutic and molecular approach. Front. Pharmacol. 2019;(977):10. doi: 10.3389/fphar.2019.00977. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

