Hyaluronic acid turnover controls the severity of cerebral cavernous malformations in bioengineered human micro-vessels
- PMID: 38352162
- PMCID: PMC10864035
- DOI: 10.1063/5.0159330
Hyaluronic acid turnover controls the severity of cerebral cavernous malformations in bioengineered human micro-vessels
Abstract
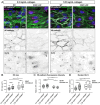
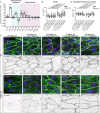
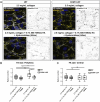
Cerebral cavernous malformations (CCMs) are vascular lesions that predominantly form in blood vessels of the central nervous system upon loss of the CCM multimeric protein complex. The endothelial cells within CCM lesions are characterized by overactive MEKK3 kinase and KLF2/4 transcription factor signaling, leading to pathological changes such as increased endothelial cell spreading and reduced junctional integrity. Concomitant to aberrant endothelial cell signaling, non-autonomous signals from the extracellular matrix (ECM) have also been implicated in CCM lesion growth and these factors might explain why CCM lesions mainly develop in the central nervous system. Here, we adapted a three-dimensional microfluidic system to examine CCM1 deficient human micro-vessels in distinctive extracellular matrices. We validate that pathological hallmarks are maintained in this model. We further show that key genes responsible for homeostasis of hyaluronic acid, a major extracellular matrix component of the central nervous system, are dysregulated in CCM. Supplementing the matrix in our model with distinct forms of hyaluronic acid inhibits pathological cell spreading and rescues barrier function. Hyaluronic acid acts by dampening cell-matrix adhesion signaling in CCM, either downstream or in parallel of KLF2/4. This study provides a proof-of-principle that ECM embedded 3D microfluidic models are ideally suited to identify how changes in ECM structure and signaling impact vascular malformations.
© 2024 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures



Similar articles
-
Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signalling.Nature. 2016 Apr 7;532(7597):122-6. doi: 10.1038/nature17178. Epub 2016 Mar 30. Nature. 2016. PMID: 27027284 Free PMC article.
-
Targeting miR-27a/VE-cadherin interactions rescues cerebral cavernous malformations in mice.PLoS Biol. 2020 Jun 5;18(6):e3000734. doi: 10.1371/journal.pbio.3000734. eCollection 2020 Jun. PLoS Biol. 2020. PMID: 32502201 Free PMC article.
-
Differential angiogenesis function of CCM2 and CCM3 in cerebral cavernous malformations.Neurosurg Focus. 2010 Sep;29(3):E1. doi: 10.3171/2010.5.FOCUS1090. Neurosurg Focus. 2010. PMID: 20809750
-
Cerebral cavernous malformations: from CCM genes to endothelial cell homeostasis.Trends Mol Med. 2013 May;19(5):302-8. doi: 10.1016/j.molmed.2013.02.004. Epub 2013 Mar 15. Trends Mol Med. 2013. PMID: 23506982 Review.
-
Role of Rho-Associated Kinase in the Pathophysiology of Cerebral Cavernous Malformations.Neurol Genet. 2024 Jan 3;10(1):e200121. doi: 10.1212/NXG.0000000000200121. eCollection 2024 Feb. Neurol Genet. 2024. PMID: 38179414 Free PMC article. Review.
Cited by
-
High-throughput differentiation of human blood vessel organoids reveals overlapping and distinct functions of the cerebral cavernous malformation proteins.Angiogenesis. 2025 Jun 6;28(3):32. doi: 10.1007/s10456-025-09985-5. Angiogenesis. 2025. PMID: 40478324 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

