Pharmacokinetic Comparison Between a Fixed-Dose Combination of Atorvastatin/Omega-3-Acid Ethyl Esters and the Corresponding Loose Combination in Healthy Korean Male Subjects
- PMID: 38352172
- PMCID: PMC10861834
- DOI: 10.2147/DDDT.S435885
Pharmacokinetic Comparison Between a Fixed-Dose Combination of Atorvastatin/Omega-3-Acid Ethyl Esters and the Corresponding Loose Combination in Healthy Korean Male Subjects
Abstract
Purpose: Statins are widely used in combination with omega-3 fatty acids for the treatment of patients with dyslipidemia. The aim of this study was to compare the pharmacokinetic (PK) profiles of atorvastatin and omega-3-acid ethyl esters between fixed-dose combination (FDC) and loose combination in healthy subjects.
Methods: A randomized, open-label, single-dose, 2-sequence, 2-treatment, 4-period replicated crossover study was performed. Subjects were randomly assigned to one of the 2 sequences and alternately received four FDC soft capsules of atorvastatin/omega-3-acid ethyl esters (10/1000 mg) or a loose combination of atorvastatin tablets (10 mg × 4) and omega-3-acid ethyl ester soft capsules (1000 mg× 4) for four periods, each period accompanied by a high-fat meal. Serial blood samples were collected for PK analysis of atorvastatin, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). PK parameters were calculated by a non-compartmental analysis. The geometric mean ratio (GMR) and its 90% confidence interval (CI) of the FDC to the loose combination were calculated to compare PK parameters.
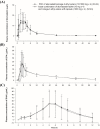
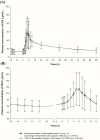
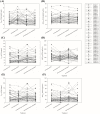
Results: A total of 43 subjects completed the study as planned. The GMR (90% CI) of FDC to loose combination for maximum concentration (Cmax) and area under the time-concentration curve from zero to the last measurable point (AUClast) were 1.0931 (1.0054-1.1883) and 0.9885 (0.9588-1.0192) for atorvastatin, 0.9607 (0.9068-1.0178) and 0.9770 (0.9239-1.0331) for EPA, and 0.9961 (0.9127-1.0871) and 0.9634 (0.8830-1.0512) for DHA, respectively. The intra-subject variability for Cmax and AUClast of DHA was 30.8% and 37.5%, respectively, showing high variability. Both the FDC and the loose combination were safe and well tolerated.
Conclusion: The FDC of atorvastatin and omega-3-acid ethyl esters showed comparable PK characteristics to the corresponding loose combination, offering a convenient therapeutic option for the treatment of dyslipidemia.
Keywords: cardiovascular disease; pharmacokinetics.
© 2024 Khwarg et al.
Conflict of interest statement
Won-Ho Kang, Youn Woong Choi, Dae Chul Ha, RaeHoon Jung, Min-Gu Han, Won Tae Jung, Kyu-Yeol Nam, and YeSeul Kim are employees of Korea United Pharm. Inc. Hye Jung Lee, Kyu Yeon Kim, Ki-Sun Jeong, and Chongho Won are employees of Caleb Multilab, Inc. The other authors do not have any conflicts of interest for this study.
Figures

References
-
- Nikos Pappan AR. Dyslipidemia. Treasure Island (FL): StatPearls Publishing; 2022. - PubMed
-
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144 - DOI - PMC - PubMed
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

