Safety and immunogenicity of VLPCOV-02, a SARS-CoV-2 self-amplifying RNA vaccine with a modified base, 5-methylcytosine
- PMID: 38352232
- PMCID: PMC10863314
- DOI: 10.1016/j.isci.2024.108964
Safety and immunogenicity of VLPCOV-02, a SARS-CoV-2 self-amplifying RNA vaccine with a modified base, 5-methylcytosine
Abstract
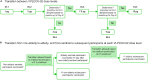
Continuing emergence of variants of concern resulting in reduced SARS-CoV-2 vaccine efficacy necessitates additional prevention strategies. The structure of VLPCOV-01, a lipid nanoparticle-encapsulated, self-amplifying RNA COVID-19 vaccine with a comparable immune response to BNT162b2, was revised by incorporating a modified base, 5-methylcytosine, to reduce reactogenicity, and an updated receptor-binding domain derived from the Brazil (gamma) variant. Interim analyses of a phase 1 dose-escalation booster vaccination study with the resulting construct, VLPCOV-02, in healthy, previously vaccinated Japanese individuals (N = 96) are reported (jRCT2051230005). A dose-related increase in solicited local and systemic adverse events was observed, which were generally rated mild or moderate. The most commonly occurring events were tenderness, pain, fatigue, and myalgia. Serum SARS-CoV-2 immunoglobulin titers increased during the 4 weeks post-immunization. VLPCOV-02 demonstrated a favorable safety profile compared with VLPCOV-01, with reduced adverse events and fewer fever events at an equivalent dose. These findings support further study of VLPCOV-02.
Keywords: Immunology; Virology.
© 2024 The Author(s).
Conflict of interest statement
M.A., D.K., T.S., K.K., Y.K., and N.S. are employees of VLP Therapeutics Japan, Inc.; K.M., M.K., and A.L.M. are employees of VLP Therapeutics, Inc.; W.A. is a board member, an employee, and holds stocks in VLP Therapeutics, Inc. and is a management board member of VLP Therapeutics Japan, Inc.; J.F.S. is an employee and holds stocks in VLP Therapeutics. Inc.; S.S. received a consultation fee from VLP Therapeutics Japan, Inc. for medical advice and consultation on clinical trial design. W.A. and J.F.S. are inventors on a related vaccine patent. The remaining authors declare no competing interest.
Figures



References
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. 2023. https://covid19.who.int/
-
- Yang Z.-R., Jiang Y.-W., Li F.-X., Liu D., Lin T.-F., Zhao Z.-Y., Wei C., Jin Q.-Y., Li X.-M., Jia Y.-X., et al. Efficacy of SARS-CoV-2 vaccines and the dose–response relationship with three major antibodies: a systematic review and meta-analysis of randomised controlled trials. Lancet. Microbe. 2023;4:e236–e246. - PMC - PubMed
-
- World Health Organization Covid19 Vaccine Tracker: 11 Vaccines Granted Emergecy Use Listing (EUL) by WHO. 2023. https://covid19.trackvaccines.org/agency/who/
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

