Whole-body galactose oxidation as a robust functional assay to assess the efficacy of gene-based therapies in a mouse model of Galactosemia
- PMID: 38352271
- PMCID: PMC10863324
- DOI: 10.1016/j.omtm.2024.101191
Whole-body galactose oxidation as a robust functional assay to assess the efficacy of gene-based therapies in a mouse model of Galactosemia
Abstract
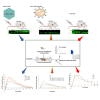
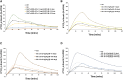
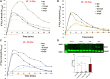
Despite the implementation of lifesaving newborn screening programs and a galactose-restricted diet, many patients with classic galactosemia develop long-term debilitating neurological deficits and primary ovarian insufficiency. Previously, we showed that the administration of human GALT mRNA predominantly expressed in the GalT gene-trapped mouse liver augmented the expression of hepatic GALT activity, which decreased not only galactose-1 phosphate (gal-1P) in the liver but also peripheral tissues. Since each peripheral tissue requires distinct methods to examine the biomarker and/or GALT effect, this highlights the necessity for alternative strategies to evaluate the overall impact of therapies. In this study, we established that whole-body galactose oxidation (WBGO) as a robust, noninvasive, and specific method to assess the in vivo pharmacokinetic and pharmacodynamic parameters of two experimental gene-based therapies that aimed to restore GALT activity in a mouse model of galactosemia. Although our results illustrated the long-lasting efficacy of AAVrh10-mediated GALT gene transfer, we found that GALT mRNA therapy that targets the liver predominantly is sufficient to sustain WBGO. The latter could have important implications in the design of novel targeted therapy to ensure optimal efficacy and safety.
Keywords: adeno-associated viral vectors; breath test; classic galactosemia; galactose oxidation; gene therapy; lipid nanoparticles; mRNA-based therapy.
© 2024 The Author(s).
Conflict of interest statement
The authors declare the following competing interests: X.Y., F.W., J.T., L.C., R.D., S.C., S.M., Y.W., M.Z., J.S., P.G.V.M., and P.F.F. are employees of Moderna and hold equities from the company.
Figures




References
-
- Isselbacher K.J., Anderson E.P., Kurahashi K., Kalckar H.M. Congenital galactosemia, a single enzymatic block in galactose metabolism. Science. 1956;123:635–636. - PubMed
-
- Fridovich-Keil J.L. Galactosemia: the good, the bad, and the unknown. J. Cell. Physiol. 2006;209:701–705. - PubMed
-
- Tyfield L., Reichardt J., Fridovich-Keil J., Croke D.T., Elsas L.J., 2nd, Strobl W., Kozak L., Coskun T., Novelli G., Okano Y., et al. Classical galactosemia and mutations at the galactose-1-phosphate uridyl transferase (GALT) gene. Hum. Mutat. 1999;13:417–430. - PubMed
-
- Berry G.T., Segal S., Gitzelmann R. In: Inborn Metabolic Diseases - Diagnosis and Treatment. Fernandes J., Saudubray M., van den Berghe G., Walter J.H., editors. Springer-Verlag; 2006. Disorders of Galactose Metabolism.
-
- Segal S., Berry G.T. In: The Metabolic Basis of Inherited Diseases. Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. McGraw-Hill; 1995. Disorders of galactose metabolism; pp. 967–1000.
Grants and funding
LinkOut - more resources
Full Text Sources

