This is a preprint.
Activation of XBP1s attenuates disease severity in models of proteotoxic Charcot-Marie-Tooth type 1B
- PMID: 38352425
- PMCID: PMC10862880
- DOI: 10.1101/2024.01.31.577760
Activation of XBP1s attenuates disease severity in models of proteotoxic Charcot-Marie-Tooth type 1B
Update in
-
Activation of XBP1s attenuates disease severity in models of proteotoxic Charcot-Marie-Tooth type 1B.Brain. 2025 Jun 3;148(6):1978-1993. doi: 10.1093/brain/awae407. Brain. 2025. PMID: 39979221 Free PMC article.
Abstract
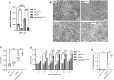
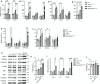
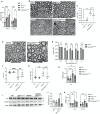
Mutations in myelin protein zero (MPZ) are generally associated with Charcot-Marie-Tooth type 1B (CMT1B) disease, one of the most common forms of demyelinating neuropathy. Pathogenesis of some MPZ mutants, such as S63del and R98C, involves the misfolding and retention of MPZ in the endoplasmic reticulum (ER) of myelinating Schwann cells. To cope with proteotoxic ER-stress, Schwann cells mount an unfolded protein response (UPR) characterized by activation of the PERK, ATF6 and IRE1α/XBP1 pathways. Previous results showed that targeting the PERK UPR pathway mitigates neuropathy in mouse models of CMT1B; however, the contributions of other UPR pathways in disease pathogenesis remains poorly understood. Here, we probe the importance of the IRE1α/XBP1 signalling during normal myelination and in CMT1B. In response to ER stress, IRE1α is activated to stimulate the non-canonical splicing of Xbp1 mRNA to generate spliced Xbp1 (Xbp1s). This results in the increased expression of the adaptive transcription factor XBP1s, which regulates the expression of genes involved in diverse pathways including ER proteostasis. We generated mouse models where Xbp1 is deleted specifically in Schwann cells, preventing XBP1s activation in these cells. We observed that Xbp1 is dispensable for normal developmental myelination, myelin maintenance and remyelination after injury. However, Xbp1 deletion dramatically worsens the hypomyelination and the electrophysiological and locomotor parameters observed in young and adult CMT1B neuropathic animals. RNAseq analysis suggested that XBP1s exerts its adaptive function in CMT1B mouse models in large part via the induction of ER proteostasis genes. Accordingly, the exacerbation of the neuropathy in Xbp1 deficient mice was accompanied by upregulation of ER-stress pathways and of IRE1-mediated RIDD signaling in Schwann cells, suggesting that the activation of XBP1s via IRE1 plays a critical role in limiting mutant protein toxicity and that this toxicity cannot be compensated by other stress responses. Schwann cell specific overexpression of XBP1s partially re-established Schwann cell proteostasis and attenuated CMT1B severity in both the S63del and R98C mouse models. In addition, the selective, pharmacologic activation of IRE1α/XBP1 signaling ameliorated myelination in S63del dorsal root ganglia explants. Collectively, these data show that XBP1 has an essential adaptive role in different models of proteotoxic CMT1B neuropathy and suggest that activation of the IRE1α/XBP1 pathway may represent a therapeutic avenue in CMT1B and possibly for other neuropathies characterized by UPR activation.
Keywords: Charcot-Marie-Tooth; Schwann cell; XBP1; demyelinating neuropathy; proteostasis; unfolded protein response.
Conflict of interest statement
Competing interests JWK and RLW are shareholders and scientific advisory board members for Protego Biopharma who have licensed IRE1/XBP1s activators including IXA4 for therapeutic development.
Figures





Similar articles
-
Activation of XBP1s attenuates disease severity in models of proteotoxic Charcot-Marie-Tooth type 1B.Brain. 2025 Jun 3;148(6):1978-1993. doi: 10.1093/brain/awae407. Brain. 2025. PMID: 39979221 Free PMC article.
-
Phosphorylation of eIF2α Promotes Schwann Cell Differentiation and Myelination in CMT1B Mice with Activated UPR.J Neurosci. 2020 Oct 14;40(42):8174-8187. doi: 10.1523/JNEUROSCI.0957-20.2020. Epub 2020 Sep 24. J Neurosci. 2020. PMID: 32973043 Free PMC article.
-
Ablation of Perk in Schwann Cells Improves Myelination in the S63del Charcot-Marie-Tooth 1B Mouse.J Neurosci. 2016 Nov 2;36(44):11350-11361. doi: 10.1523/JNEUROSCI.1637-16.2016. J Neurosci. 2016. PMID: 27807175 Free PMC article.
-
Navigating the Landscape of CMT1B: Understanding Genetic Pathways, Disease Models, and Potential Therapeutic Approaches.Int J Mol Sci. 2024 Aug 26;25(17):9227. doi: 10.3390/ijms25179227. Int J Mol Sci. 2024. PMID: 39273178 Free PMC article. Review.
-
Roles of XBP1s in Transcriptional Regulation of Target Genes.Biomedicines. 2021 Jul 8;9(7):791. doi: 10.3390/biomedicines9070791. Biomedicines. 2021. PMID: 34356855 Free PMC article. Review.
References
-
- Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952. - PubMed
-
- Giese KP, Martini R, Lemke G, Soriano P, Schachner M. Mouse P0 gene disruption leads to hypomyelination, abnormal expression of recognition molecules, and degeneration of myelin and axons. Cell. 1992;71(4):565–576. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
