This is a preprint.
Detailing organelle division and segregation in Plasmodium falciparum
- PMID: 38352445
- PMCID: PMC10862848
- DOI: 10.1101/2024.01.30.577899
Detailing organelle division and segregation in Plasmodium falciparum
Update in
-
Detailing organelle division and segregation in Plasmodium falciparum.J Cell Biol. 2024 Dec 2;223(12):e202406064. doi: 10.1083/jcb.202406064. Epub 2024 Nov 1. J Cell Biol. 2024. PMID: 39485315 Free PMC article.
Abstract
The malaria causing parasite, Plasmodium falciparum, replicates through a tightly orchestrated process termed schizogony, where approximately 32 daughter parasites are formed in a single infected red blood cell and thousands of daughter cells in mosquito or liver stages. One-per-cell organelles, such as the mitochondrion and apicoplast, need to be properly divided and segregated to ensure a complete set of organelles per daughter parasites. Although this is highly essential, details about the processes and mechanisms involved remain unknown. We developed a new reporter parasite line that allows visualization of the mitochondrion in blood and mosquito stages. Using high-resolution 3D-imaging, we found that the mitochondrion orients in a cartwheel structure, prior to stepwise, non-geometric division during the last stage of schizogony. Analysis of focused ion beam scanning electron microscopy (FIB-SEM) data confirmed these mitochondrial division stages. Furthermore, these data allowed us to elucidate apicoplast division steps, highlighted its close association with the mitochondrion, and showed putative roles of the centriolar plaques (CPs) in apicoplast segregation. These observations form the foundation for a new detailed mechanistic model of mitochondrial and apicoplast division and segregation during P. falciparum schizogony and pave the way for future studies into the proteins and protein complexes involved in organelle division and segregation.
Figures

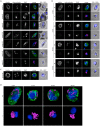
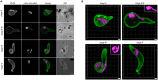
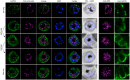
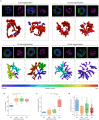


References
-
- WHO. World malaria report 2023. (2023).
-
- Vaidya A. B. & Mather M. W. Mitochondrial evolution and functions in malaria parasites. Annu. Rev. Microbiol. 63, 249–267 (2009). - PubMed
-
- Painter H. J., Morrisey J. M., Mather M. W. & Vaidya A. B. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature 446, 88–91 (2007). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
