This is a preprint.
Bacterium secretes chemical inhibitor that sensitizes competitor to bacteriophage infection
- PMID: 38352521
- PMCID: PMC10862869
- DOI: 10.1101/2024.01.31.578241
Bacterium secretes chemical inhibitor that sensitizes competitor to bacteriophage infection
Update in
-
Streptomyces secretes a siderophore that sensitizes competitor bacteria to phage infection.Nat Microbiol. 2025 Feb;10(2):362-373. doi: 10.1038/s41564-024-01910-8. Epub 2025 Jan 8. Nat Microbiol. 2025. PMID: 39779880
Abstract
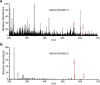
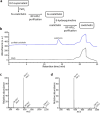
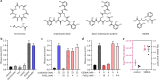
To overtake competitors, microbes produce and secrete secondary metabolites that kill neighboring cells and sequester nutrients. This natural product-mediated competition likely evolved in complex microbial communities that included viral pathogens. From this ecological context, we hypothesized that microbes secrete metabolites that "weaponize" natural pathogens (i.e., bacteriophages) to lyse their competitors. Indeed, we discovered a bacterial secondary metabolite that sensitizes other bacteria to phage infection. We found that this metabolite provides the producer (a Streptomyces sp.) with a fitness advantage over its competitor (Bacillus subtilis) by promoting phage infection. The phage-promoting metabolite, coelichelin, sensitized B. subtilis to a wide panel of lytic phages, and it did so by preventing the early stages of sporulation through iron sequestration. Beyond coelichelin, other natural products may provide phage-mediated competitive advantages to their producers-either by inhibiting sporulation or through yet-unknown mechanisms.
Figures





Similar articles
-
Streptomyces secretes a siderophore that sensitizes competitor bacteria to phage infection.Nat Microbiol. 2025 Feb;10(2):362-373. doi: 10.1038/s41564-024-01910-8. Epub 2025 Jan 8. Nat Microbiol. 2025. PMID: 39779880
-
Linearmycins Activate a Two-Component Signaling System Involved in Bacterial Competition and Biofilm Morphology.J Bacteriol. 2017 Aug 22;199(18):e00186-17. doi: 10.1128/JB.00186-17. Print 2017 Sep 15. J Bacteriol. 2017. PMID: 28461449 Free PMC article.
-
Repeated outbreaks drive the evolution of bacteriophage communication.Elife. 2021 Jan 18;10:e58410. doi: 10.7554/eLife.58410. Elife. 2021. PMID: 33459590 Free PMC article.
-
Phage therapy: From biological mechanisms to future directions.Cell. 2023 Jan 5;186(1):17-31. doi: 10.1016/j.cell.2022.11.017. Cell. 2023. PMID: 36608652 Free PMC article. Review.
-
Understanding bacteriophage specificity in natural microbial communities.Viruses. 2013 Mar 11;5(3):806-23. doi: 10.3390/v5030806. Viruses. 2013. PMID: 23478639 Free PMC article. Review.
References
-
- Friedrich M. W., Microbial Communities, Structure, and Function. In Encyclopedia of Geobiology, Reitner J.; Thiel V., Eds. Springer Netherlands: Dordrecht, 2011; p 592–595.
-
- Ghoul M.; Mitri S. The Ecology and Evolution of Microbial Competition. Trends in Microbiology 2016, 24 (10), 833–845. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
