This is a preprint.
Age-Related RPE changes in Wildtype C57BL/6J Mice between 2 and 32 Months
- PMID: 38352604
- PMCID: PMC10862734
- DOI: 10.1101/2024.01.30.574142
Age-Related RPE changes in Wildtype C57BL/6J Mice between 2 and 32 Months
Abstract
Purpose: This study provides a systematic evaluation of age-related changes in RPE cell structure and function using a morphometric approach. We aim to better capture nuanced predictive changes in cell heterogeneity that reflect loss of RPE integrity during normal aging. Using C57BL6/J mice ranging from P60-P730, we sought to evaluate how regional changes in RPE shape reflect incremental losses in RPE cell function with advancing age. We hypothesize that tracking global morphological changes in RPE is predictive of functional defects over time.
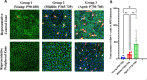
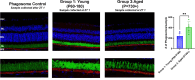
Methods: We tested three groups of C57BL/6J mice (young: P60-180; Middle-aged: P365-729; aged: 730+) for function and structural defects using electroretinograms, immunofluorescence, and phagocytosis assays.
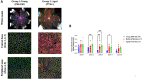
Results: The largest changes in RPE morphology were evident between the young and aged groups, while the middle-aged group exhibited smaller but notable region-specific differences. We observed a 1.9-fold increase in cytoplasmic alpha-catenin expression specifically in the central-medial region of the eye between the young and aged group. There was an 8-fold increase in subretinal, IBA-1-positive immune cell recruitment and a significant decrease in visual function in aged mice compared to young mice. Functional defects in the RPE corroborated by changes in RPE phagocytotic capacity.
Conclusions: The marked increase of cytoplasmic alpha-catenin expression and subretinal immune cell deposition, and decreased visual output coincide with regional changes in RPE cell morphometrics when stratified by age. These cumulative changes in the RPE morphology showed predictive regional patterns of stress associated with loss of RPE integrity.
Figures






Similar articles
-
Wheel running exercise protects against retinal degeneration in the I307N rhodopsin mouse model of inducible autosomal dominant retinitis pigmentosa.Mol Vis. 2019 Aug 21;25:462-476. eCollection 2019. Mol Vis. 2019. PMID: 31523123 Free PMC article.
-
Differences in the distribution, phenotype and gene expression of subretinal microglia/macrophages in C57BL/6N (Crb1 rd8/rd8) versus C57BL6/J (Crb1 wt/wt) mice.J Neuroinflammation. 2015 Jan 15;12:6. doi: 10.1186/s12974-014-0221-4. J Neuroinflammation. 2015. PMID: 25588310 Free PMC article.
-
Mitochondria dynamics in the aged mice eye and the role in the RPE phagocytosis.Exp Eye Res. 2021 Dec;213:108800. doi: 10.1016/j.exer.2021.108800. Epub 2021 Oct 21. Exp Eye Res. 2021. PMID: 34688622
-
Functional principal component analysis reveals discriminating categories of retinal pigment epithelial morphology in mice.Invest Ophthalmol Vis Sci. 2013 Nov 5;54(12):7274-83. doi: 10.1167/iovs.13-12450. Invest Ophthalmol Vis Sci. 2013. PMID: 24114543 Free PMC article.
-
No Difference Between Age-Matched Male and Female C57BL/6J Mice in Photopic and Scotopic Electroretinogram a- and b-Wave Amplitudes or in Peak Diurnal Outer Segment Phagocytosis by the Retinal Pigment Epithelium.Adv Exp Med Biol. 2019;1185:507-511. doi: 10.1007/978-3-030-27378-1_83. Adv Exp Med Biol. 2019. PMID: 31884662 Free PMC article.
References
-
- Pauleikhoff D, Harper CA, Marshall J, Bird AC. Aging Changes in Bruch’s Membrane: A Histochemical and Morphologic Study. Ophthalmology [Internet]. 1990. Feb 1 [cited 2023 Jul 16];97(2):171–8. Available from: http://www.aaojournal.org/article/S0161642090326192/fulltext - PubMed
-
- Gullapalli VK, Sugino IK, Van Patten Y, Shah S, Zarbin MA. Impaired RPE survival on aged submacular human Bruch’s membrane. Exp Eye Res. 2005. Feb;80(2):235–48. - PubMed
-
- Feeney-Burns L, Ellersieck MR. Age-Related Changes in the Ultrastructure of Bruch’s Membrane. Am J Ophthalmol. 1985. Nov 1;100(5):686–97. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
