Ubiquitin specific peptidase (USP37) mediated effects in microscaffold-encapsulated cells: a comprehensive study on growth, proliferation and EMT
- PMID: 38352690
- PMCID: PMC10862100
- DOI: 10.1039/d3ra08786g
Ubiquitin specific peptidase (USP37) mediated effects in microscaffold-encapsulated cells: a comprehensive study on growth, proliferation and EMT
Abstract
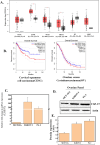
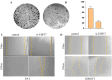
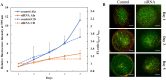
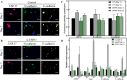
Though significant advances have been made in developing therapeutic strategies for cancer, suitable in vitro models for mechanistically identifying relevant drug targets and understanding disease progression are still lacking. Most studies are generally performed using two-dimensional (2D) models, since these models can be readily established and allow high throughput assays. However, these models have also been reported as the reason for unreliable pre-clinical information. To avoid this discrepancy, three-dimensional (3D) cell culture models have been established and have demonstrated the potential to provide alternative ways to study tissue behavior. However, most of these models first require optimization and cell cultures with a certain density, thus adding a prepping step in the platform before it can be used for any studies. This limits their use in studies where the fundamental understanding of biological processes must be carried out in a short time frame. In this study, we developed a 3D cell culture system that tests a less explored cancer therapeutic target-the deubiquitinating enzyme ubiquitin specific peptidase 37 (USP37)-in different cancer cell lines using sensitive carbon dot pH nanosensors, which provides a rapid model for studies compared to the parallel model available commercially. This enzyme is found to be elevated in different cancers and has been reported to play a role in cell cycle regulation, oncogenesis and metastasis. However, the confirmation of the role of USP37 downregulation in cellular proliferation via appropriate in vitro 3D models has not been demonstrated. To establish the applicability of the developed 3D platform in studying such oncogenes, classical 2D models have been used in this study for identifying the role of USP37 in tumor progression and metastasis. The data clearly suggests that this ingeniously developed 3D cell culture system is a better alternative to 2D models to study the growth and migration of different cancer cell lines on depletion of oncogenic proteins like USP37 and its effect on epithelial-mesenchymal transition (EMT) markers, and it can further be targeted as a viable therapeutic option.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

Similar articles
-
Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial-mesenchymal transition and cisplatin sensitivity.J Exp Clin Cancer Res. 2018 Nov 27;37(1):287. doi: 10.1186/s13046-018-0934-9. J Exp Clin Cancer Res. 2018. PMID: 30482232 Free PMC article.
-
USP37 promotes angiogenesis and metastasis in colorectal cancer by facilitating β-catenin stability.Am J Cancer Res. 2023 Jun 15;13(6):2323-2341. eCollection 2023. Am J Cancer Res. 2023. PMID: 37424824 Free PMC article.
-
Ubiquitin specific peptidase 37 and PCNA interaction promotes osteosarcoma pathogenesis by modulating replication fork progression.J Transl Med. 2023 Apr 28;21(1):286. doi: 10.1186/s12967-023-04126-2. J Transl Med. 2023. PMID: 37118828 Free PMC article.
-
Ubiquitin-specific peptidase 37: an important cog in the oncogenic machinery of cancerous cells.J Exp Clin Cancer Res. 2021 Nov 10;40(1):356. doi: 10.1186/s13046-021-02163-7. J Exp Clin Cancer Res. 2021. PMID: 34758854 Free PMC article. Review.
-
Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions?Int J Mol Sci. 2018 Jan 18;19(1):181. doi: 10.3390/ijms19010181. Int J Mol Sci. 2018. PMID: 29346265 Free PMC article. Review.
References
-
- van Duinen V. et al., Microfluidic 3D cell culture: from tools to tissue models. Curr. Opin. Biotechnol. 2015;35:118–126. - PubMed
LinkOut - more resources
Full Text Sources

