Advances in the study of marketed antibody-drug Conjugates (ADCs) for the treatment of breast cancer
- PMID: 38352694
- PMCID: PMC10862125
- DOI: 10.3389/fphar.2023.1332539
Advances in the study of marketed antibody-drug Conjugates (ADCs) for the treatment of breast cancer
Abstract
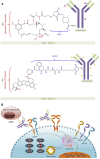
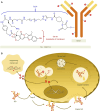
Breast cancer continues to have a high incidence rate among female malignancies. Despite significant advancements in treatment modalities, the heterogeneous nature of breast cancer and its resistance to various therapeutic approaches pose considerable challenges. Antibody-drug conjugates (ADCs) effectively merge the specificity of antibodies with the cytotoxicity of chemotherapeutic agents, offering a novel strategy for precision treatment of breast cancer. Notably, trastuzumab emtansine (T-DM1) has provided a new therapeutic option for HER2-positive breast cancer patients globally, especially those resistant to conventional treatments. The development of trastuzumab deruxtecan (T-DXd) and sacituzumab govitecan (SG) has further broadened the applicability of ADCs in breast cancer therapy, presenting new hopes for patients with low HER2 expression and triple-negative breast cancer. However, the application of ADCs presents certain challenges. For instance, their treatment may lead to adverse reactions such as interstitial lung disease, thrombocytopenia, and diarrhea. Moreover, prolonged treatment could result in ADCs resistance, complicating the therapeutic process. Economically, the high costs of ADCs might hinder their accessibility in low-income regions. This article reviews the structure, mechanism of action, and clinical trials of commercially available ADCs for breast cancer treatment, with a focus on the clinical trials of the three drugs, aiming to provide insights for clinical applications and future research.
Keywords: ADC; SG; T-DM1; T-DXd; breast cancer.
Copyright © 2024 Liang, Zhang, Li, Lai, Qi and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Sequencing Antibody Drug Conjugates in Breast Cancer: Exploring Future Roles.Curr Oncol. 2023 Nov 29;30(12):10211-10223. doi: 10.3390/curroncol30120743. Curr Oncol. 2023. PMID: 38132377 Free PMC article. Review.
-
Gastrointestinal Toxicity of Antibody Drug Conjugates (ADCs) in Metastatic Breast Cancer: A Pooled Analysis.Clin Breast Cancer. 2024 Jul;24(5):411-420. doi: 10.1016/j.clbc.2024.04.003. Epub 2024 Apr 8. Clin Breast Cancer. 2024. PMID: 38734491 Review.
-
Next-generation antibody-drug conjugates for breast cancer: Moving beyond HER2 and TROP2.Crit Rev Oncol Hematol. 2023 Oct;190:104090. doi: 10.1016/j.critrevonc.2023.104090. Epub 2023 Aug 9. Crit Rev Oncol Hematol. 2023. PMID: 37562695 Review.
-
Antibody-drug conjugates in breast cancer: current evidence and future directions.Exp Hematol Oncol. 2025 Mar 20;14(1):41. doi: 10.1186/s40164-025-00632-9. Exp Hematol Oncol. 2025. PMID: 40114224 Free PMC article. Review.
-
Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions.Breast Cancer Res. 2021 Aug 11;23(1):84. doi: 10.1186/s13058-021-01459-y. Breast Cancer Res. 2021. PMID: 34380530 Free PMC article. Review.
Cited by
-
Antibody-Drug Conjugates (ADCs): current and future biopharmaceuticals.J Hematol Oncol. 2025 Apr 30;18(1):51. doi: 10.1186/s13045-025-01704-3. J Hematol Oncol. 2025. PMID: 40307936 Free PMC article. Review.
-
Robust hierarchical co-clustering for exploring toxicogenomic biomarkers and their chemical regulators.Sci Rep. 2025 May 14;15(1):16676. doi: 10.1038/s41598-025-99568-7. Sci Rep. 2025. PMID: 40369321 Free PMC article.
-
HER2-positive gastric cancer: from targeted therapy to CAR-T cell therapy.Front Immunol. 2025 Mar 13;16:1560280. doi: 10.3389/fimmu.2025.1560280. eCollection 2025. Front Immunol. 2025. PMID: 40181988 Free PMC article. Review.
-
Trastuzumab Deruxtecan in Previously Treated HER2-Low Metastatic Breast Cancer: Real-World Multicentric Study in the Portuguese Population.Cancers (Basel). 2025 Jun 9;17(12):1911. doi: 10.3390/cancers17121911. Cancers (Basel). 2025. PMID: 40563562 Free PMC article.
-
History of trastuzumab: a case study in health technology reassessment and natural disinvestment in Veneto Region.Front Pharmacol. 2024 Aug 6;15:1406351. doi: 10.3389/fphar.2024.1406351. eCollection 2024. Front Pharmacol. 2024. PMID: 39166105 Free PMC article. No abstract available.
References
-
- Abraham J., Montero A. J., Jankowitz R. C., Salkeni M. A., Beumer J. H., Kiesel B. F., et al. (2019). Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP foundation trial FB-10. J. Clin. Oncol. 37 (29), 2601–2609. PubMed PMID: 31442103. PMCID: PMC6784849. Epub 20190823. eng. 10.1200/JCO.19.00858 - DOI - PMC - PubMed
-
- Abuhelwa Z., Alloghbi A., Alqahtani A., Nagasaka M. (2022). Trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis in ERBB2-positive advanced solid malignancies: a systematic review. Drugs 82 (9), 979–987. PubMed PMID: 35759121. PMCID: PMC9276583. Epub 20220627. eng. 10.1007/s40265-022-01736-w - DOI - PMC - PubMed
-
- André F., Hee Park Y., Kim S. B., Takano T., Im S. A., Borges G., et al. (2023). Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet 401 (10390), 1773–1785. PubMed PMID: 37086745. Epub 20230420. eng. 10.1016/S0140-6736(23)00725-0 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous